Analytical methods for the determination of mercury species in natural gas condensate
-
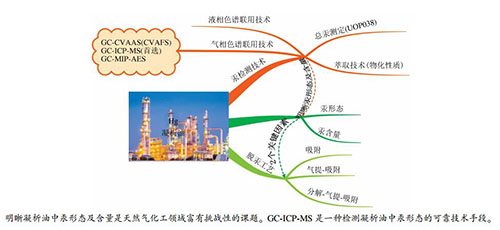
摘要: 天然气凝析油是优质石脑油原料,作为天然气的伴生品,其中,含有少量的汞及其化合物。汞不仅危害人体健康,而且在凝析油的加工转化过程中还会腐蚀热交换器等单元设备,进而引起生产安全隐患。明晰凝析油中汞的存在方式及形态演变,是天然气化工领域一个富有挑战性的课题。本文系统介绍天然气凝析油中汞的化学形态及分析技术,详细比较和讨论了凝析油等液烃中汞的萃取工艺及汞形态的检测手段,综合评价了不同检测技术的优缺点。已有研究结果表明,采用气相色谱-电感耦合等离子体质谱联用技术,汞回收率接近100%,汞的测定精度高,是一种检测凝析油等液烃中汞组成及化学形态的可靠技术手段。此外,凝析油中汞的形态及其含量是确定脱汞工艺、装置规格及脱汞剂的两个关键因素,而研发一套高精度的快速检测汞形态的新方法,是天然气化工领域需要关注的一个新方向。
-
关键词:
- 凝析油 /
- 汞形态 /
- 检测技术 /
- 气相色谱-电感耦合等离子体质谱联用技术
Abstract: Natural gas condensate, a by-product in natural gas exploitation and utilization, is an excellent raw material for naphtha production. However, the natural gas condensate always contains a trace amount of mercury, which may damage the human health and corrode the downstream processing units such as heat exchangers. It is highly demanded that the mercury in natural gas condensate can be identified specifically and analyzed accurately and quickly, which remains a big challenge in natural gas processing industry up to now. This paper reviewed the analytical methods for the determination of mercury species in natural gas condensate, by evaluating the advantages and disadvantages of various measures in terms of mercury extraction and detection in natural gas condensate. It was observed that the gas chromatography-inductively coupled plasma-mass spectrometer with high accuracy and high mercury recovery rate is the best technique at present to determine the mercury species in the natural gas condensate. The state of mercury species and content of mercury are two key factors to govern the selection and optimization of the adsorbent and the process for the efficient removal of mercury in natural gas condensate in different scales, which exacts a novel technique for the fast yet accurate characterization of various mercury species to meet the demands in the natural gas industry. -
表 1 部分地区天然气和凝析油中汞含量[9]
Table 1 Mercury contents in the natural gas and natural gas condensate from different regions[9]
Region Natural gas/(μg·m-3) Natural gas condensate/(μg·kg-1) Europe 100-150 ND South America 50-120 50-100 Gulf of Thailand 100-400 400-1200 Africa 80-100 500-1000 North Africa 50-80 20-50 Malaysia 1-200 10-100 Indonesia 200-300 10-500 ND: not detected 表 2 煤、石油、天然气和凝析油中汞化合物的自然丰度
Table 2 Natural abundance of mercury species in coal, crude oil, natural gas and natural gas condensate
Mercury species Coal Crude oil Natural gas Natural gas condensate Hg0 ≤1% main main main (CH3)2Hg NS ≤1% ≤1% ≤1% HgCl2 10%-50% 10%-50% ND 10%-50% HgS NS solid particles ND solid particles HgO ≤1% ND ND ND CH3HgCl NS ≤1% ND ≤1% NS: not sure; ND: not detected 表 3 Bloom等对凝析油和原油中的汞形态测定结果(ng/g)[19]
Table 3 Mercury species in natural gas condensate and crude oil (ng/g)[19]
Sample Unfiltered mercury 0.8 μm nitrocellulose membrane filtered mercury total Hg0 dissolved Hg2+ CH3Hg Natural gas #1 20700 3060 5210 2150 3.74 condensate #2 49400 34500* 36800 237 6.24 Crude oil #1 1990 408 821 291 0.25 #2 4750 1120 1470 433 0.26 #3 4610 536 1680 377 0.27 #4 4100 1250 1770 506 0.62 #5 15200 2930 3110 489 0.45 #6 1.51 0.09 1.01 0.39 0.15 #7 0.42 0.17 0.41 0.02 0.11 *: this sample contained particulate Hg0 that was re-dissolved in hexane 表 4 不同凝析样品油中汞化合物含量(μg/L)[20]
Table 4 ontents of mercury compound in various natural gas condensates (μg/L)[20]
Sample Injection model Hg0 HgCl2 DMeHg MeEtHg DEtHg MeHgCl EtHgCl Total Organic mercury ratio Condensate #1 pulsed splitless ND 30.9 < < < < < 32.4 - on-column 1.53 ND < < < < < #2 pulsed splitless ND 8.13 2.06 3.2 0.86 0.21 0.05 15.1 42% on-column 0.52 ND 1.99 3.2 1.01 < < #3 pulsed splitless ND 116 9.23 13.1 5.07 0.32 < 173 16% on-column 28.8 ND 9.31 14.9 5.18 < < #4 pulsed splitless ND 7.44 0.78 0.97 0.11 0.09 < 12.2 16% on-column 2.66 ND 1.1 1.03 0.1 < < #5 pulsed splitless ND 26.8 < < < < < 27.7 - on-column 0.86 ND < < < < < #6* pulsed splitless ND 48.4 < < < < < 48.4 - on-column < ND < < < < < #7* pulsed splitless ND 0.68 23.4 29.1 5.81 0.51 < 59.5 99% on-column < ND 21.4 28.6 6.28 < < #8* pulsed splitless ND 0.14 3.98 3.19 0.14 0.13 0.07 7.68 98% on-column 0.03 ND 3.91 2.98 0.21 < < Crude oil #9 pulsed splitless ND 0.60 < < < < < 0.63 on-column 0.03 ND < < < < < ND: not detected; < : below detection limit; the average values of both injection models were adopted for DMeHg, MeEtHg and DEtHg;
*: condensate naphtha;-: not calculated表 5 凝析油中汞化合物的不同检测方法的检测限
Table 5 Detection limit of various methods for mercury speciation in natural gas condensate
Mercury species Limit of detection /(μg·L-1) Ref. Hg0 Hg2+ MeEtHg MeHg+ (MeHg) EtHg+ (EtHg) Me2Hg MePrHg Et2Hg MeBuHg Pr2Hg Bu2Hg GC-MIP-AES ND 0.56 ND 0.56 ND 0.24 ND ND ND ND ND [27] 0.98 ND ND ND ND 1.32 0.16 0.08 0.14 0.20 0.11 [26] GC-ICP-MS 0.15 0.34 0.019 0.074 0.05 0.2 ND 0.035 ND ND 0.05 [20] ND 0.03 ND 0.09 ND ND ND ND ND ND ND [36] HPLC-CVAFS ND ND ND 0.05 0.07 ND ND ND ND ND ND [39] ND: not detected 表 6 不同检测方法对凝析油或原油中形态汞的汞回收率以及相对标准偏差的影响
Table 6 Mercury recovery rate and relative standard deviation of mercury species in natural gas condensate or crude oil analyzed by various methods
Analysis method MeHg EtHg DMeHg DEtHg MeHgCl EtHgCl Hg2+ Hg0 Ref. CVAFS mercury recovery rate /% 105.5 ND ND ND ND ND 93.1 98.7 [19] GC-MIP-AES mercury recovery rate /% ND ND 94.7 ND 108.9 ND 99.2 ND [27] GC-ICP-MS(a) mercury recovery rate /% ND ND 168.9 99.9 103.1 100.1 114 ND RSD /% ND ND 3.2 3.0 2.6 1.6 3.2 3.4 GC-ICP-MS(b) mercury recovery rate /% ND ND 223 90.2 94.9 92.1 ND 77.5 RSD /% ND ND 1.6 (5.1c) 2.5 (4.4c) 2.7 2.6 ND 2.2 (3.9c) [20] HPLC-CVAFS mercury recovery rate /% 86.7 70.6 ND ND ND ND ND ND [39] RSD(%) 1.8 2.1 ND ND ND ND ND ND a: pulsed splitless injection mode; b: on-column injection mode; c: crude oil or condensate gas Process Solid adsorption Decomposition-adsorption Decomposition-stripping-adsorption Total mercury content medium high higher Organic mercury lower high higher Mercury adsorbents Ag molecular sieve, metal iodide & metal sulfide sorbent Ag, S or metal sulfide sorbent metal sulfide sorbent Catalyst - Pt, Pd(temp. 160-200 ℃) Pt, Pd(temp. 160-200 ℃) Stripping gas - - natural gas & air Characteristics simple process, mature technology a little more complicated process, medium cost complex process, high cost Atmosphere dry gas dry gas dry gas & wet gas Table 8 Comprehensive evaluation of adsorbent for removal of mercury from natural gas condensate[17, 42]
Adsorbents Metal halide adsorbent Ag Molecular sieve Metal sulfide adsorbent(CuS) Regeneration non-regenerable regenerable non-regenerable Atmosphere dry gas dry gas & wet gas dry gas & wet gas Secondary pollution with without without Applicability Hg0, Hg2+ & organic mercury Hg0 Hg0, part of organic mercury stability low high medium Industrialization low light hydrocarbon high -
[1] MAC KINNON M A, BROUWER J, SAMUELSEN S. The role of natural gas and its infrastructure in mitigating greenhouse gas emissions, improving regional air quality, and renewable resource integration[J]. Prog Energy Combust Sci, 2018, 64: 62-92. [2] PANG X Q, JIA C Z, WANG W Y. Petroleum geology features and research developments of hydrocarbon accumulation in deep petroliferous basins[J]. Pet Sci, 2015, 12: 1-53. [3] 韩中喜, 王淑英, 严启团, 葛守国, 王春怡.松辽盆地双坨子气田天然气汞含量特征[J].科技导报, 2015, 33: 40-44.HAN Zhong-xi, WANG Shu-ying, YAN Qi-tuan, GE Shou-guo, WANG Chun-yi. Analysis of natural gas mercury content characteristics of Shuangtuozi gas field in Songliao Basin[J]. Sci Technol Rev, 2015, 33: 40-44. [4] 刘全有, 李剑, 侯路.油气中汞及其化合物样品采集与实验分析方法研究进展[J].天然气地球科学, 2006, 14(7): 559-565.LIU Quan-you, LI Jian, HOU Lu. Advance of research on mercury and its compounds collecting and measuring methods[J]. Nat Gas Geosci, 2006, 14(7): 559-565. [5] 薛艳.石油中汞的分析方法进展[J].当代石油化学, 2008, 16(6): 33-35.XUE Yan. Research development of mercury analysis method in petroleum[J]. Pet Petrochem Today, 2008, 16(6): 33-35. [6] FUTSAETER G, WILSON The UNEP global mercury assessment: Sources, emissions and transport[C]. E3S Web of Conferences, 2012, Rome, Italy. [7] WILHELM S M. Avoiding exposure to mercury during inspection and maintenance operations in oil and gas processing[J]. Process Saf Prog, 1999, 18: 178-188. [8] KEHAL M, MENNOUR A, REINERT L, FUZELLIER H. Heavy metals in water if the Skikda Bay Les Metaux Lourds dans les Eaux de la Baie De Skikda[J]. Environ Technol, 2004, 25: 1059-1065. [9] 山田淳也, 川崎绿, 大塚町惠, 金田英伯.原油·天然ガス生産における水銀への対応[J].石油技術協会誌, 2016, 81(5): 401-407。YAMADA J, KAYASAKI M, OTSUKA M, KANETA H. Handing of mercury issues on oil and gas production[J]. J Japn Assoc Perol Technology, 2016, 81(5): 401-407. [10] GAULIER F, GIBERT A, WALLS D, LANGFORD M, BAKER S, PORCHERON A F, LIENEMANN C P. Mercury speciation in liquid petroleum products: Comparison between on-site approach and lab measurement using size exclusion chromatography with high resolution inductively coupled plasma mass spectrometric detection (SEC-ICP-HR MS)[J]. Fuel Process Technol, 2015, 131: 254-261. [11] JESUS A D, ZMOZINSKI A V, VIEIRA M A, RIBEIRO A S, SILVA M M. Determination of mercury in naphtha and petroleum condensate by photochemical vapor generation atomic absorption spectrometry[J]. Microchem J, 2013, 110: 227-232. [12] SALVA A C, GALLUP D L. Mercury removal process is applied to crude oil of southern Argentina[C]. SPE Latin American and Caribbean Petroleum Conference, 2010, Lima, Peru. [13] WILHELM S M, LIANG L, CUSSEN D, KIRCHGESSNER D A. Mercury in crude oil processed in the United States (2004)[J]. Environ Sci Technol, 2007, 41: 4509-4514. [14] BOUYSSIERE B, BACO F, SAVARY L, LOBINSKI R. Analytical methods for speciation of mercury in gas condensate[J]. Oil Gas Sci Technol-Rev IFP, 2000, 55: 639-648. [15] FINSTER M E, RAYMOND M R, SCOFIELD M A, SMITH K. Mercury-impacted scrap metal: Source and nature of the mercury[J]. J Envrion Manag, 2015, 161: 303-308. [16] DOLL B, KNICKERBOCKER B M, NUCCI E. Industry response to the UN global mercury treaty negotiations focus on oil and gas[C]. International Conference on Health, Safety and Environment in Oil and Gas Exploration and Production, 2012, Perth, Australia. [17] 张哲, 梁金川, 黄永恒.凝析油脱汞工艺分析[J].石油化工应用, 2011, 30(12): 88-90, 104.ZHANG Zhe, LIANG Jin-chuan, HUANG Yong-heng. Study on processes of mercury removal from gas condensate[J]. Petrochem Ind Appl, 2011, 30(12): 88-90, 104. [18] 王卫平, 王子军.石油和天然气中汞的赋存状态及其脱除方法[J].石油化工腐蚀与防护, 2010, 27(3): 1-4.WANG Wei-ping, WANG Zi-jun. Species and removal methods of mercury in petroleum and natural gas[J]. Corros Protect Petroch Ind, 2010, 27(3): 1-4. [19] BLOOM N S. Analysis and stability of mercury speciation in petroleum hydrocarbons[J]. Fresenius J Anal Chem, 2000, 366: 438-443. [20] TAO H, MURAKIMI T, TOMINAGA M, MIYAZAKI A. Mercury speciation in natural gas condensate by gas chromatography-inductively coupled plasma mass spectrometry[J]. J Anal Atom Spectrom, 1998, 13: 1085-1093. [21] UOP L L C, DES PLAINES I L. 2010: Total mercury and mercury species in liquid hydrocarbons[S]. UOP Method 938-10. [22] FRECH W, BAXTER D C, BAKKLE B, SNELL J, THOMASSEN Y. Determination and speciation of mercury in natural gases and gas condensates[J]. Anal Commun, 1996, 33: 7H-9H. [23] FURUTA A, SATO X, TAKAHASHI K. Trace analysis of mercury compounds in natural gas condensate[C]. Process of the International Trace Analysis Symposium, 1992, Sendai and Kiryu, Japan. [24] SNELL J, QIAN J, JOHANSSON M, SMIT K, FRECH W. Stability and reaction of mercury species in organic solution[J]. The Analyst, 1998, 123: 905-909. [25] BLOOM N. Determination of picogram levels of methylmercury by aqueous phase ethylation, followed by cryogenic gas chromatography with cold vapour atomic fluorescence detection[J]. Can J Fish Aquat Sci, 1989, 46: 1131-1140. [26] BOUYSSIERE B, BACO F, SAVARY L, LOBINSKI R. Speciation analysis for mercury in gas condensates by capillary gas chromatography with inductively coupled plasma mass spectrometric detection[J]. J Chromatogr A, 2002, 976: 431-439. [27] SNELL J P, FRECH W, THOMASSEN Y. Performance improvements in the determination of mercury species in natural gas condensate using an on-line amalgamation trap or solid-phase micro-extraction with capillary gas chromatography-microwave-induced plasma atomic emission spectrometry[J]. Analyst, 1996, 121: 1055-1060. [28] BULSKA E, BAXTER D C, FRECH W. Capillary column gas chromatography for mercury speciation[J]. Anal Chim Acta, 1991, 12: 545-554. [29] MIZUISHI K, TAKEUCHI M, HOBO T. Direct GC determination of methylmercury chloride on HBr-methanol-treated capillary columns[J]. Chromatographia, 1997, 44(7/8): 386-392. [30] KATO T, UEHIRO T, YASUHARA A, MORITA M. Determination of methylmercury species by capillary column gas chromatography with axially viewed inductively coupled plasma atomic-emission-spectrometric detection[J]. J Anal At Spectrom, 1992, 7: 15. [31] BARSHICK C M, BARSHICK S A, WALSH E B, VANCE M A, BRITT P F. Application of isotope dilution to ion trap gas chromatography/mass spectrometry[J]. Anal Chem 1999, 71: 483-488. [32] LANSENS P, MEULEMAN C, LEERMAKERS M, BAEYENS W. Determination of methylmercury in natural waters by headspace gas chromatography with microwave induced plasma detection after preconcentration on resin containing dithiocarbamate groups[J]. Anal Chim Acta, 1990, 234: 417-424. [33] LOBINSKI R, ADMAS F C. Speciation analysis by gas chromatography with plasma source spectrometric detection[J]. Spectrochimica Acta, Part B, 1997, 52B: 1865-1903. [34] SULLIVAN J J, QUIMBY B D. Characterization of computerized photodiode array spectrometer for gas chromatography-atomic emission spectrometry[J]. Anal Chem, 1990, 62: 1034-1043. [35] QUIMBY B D, SULLIVAN J J. Evaluation of microwave cavity, discharge tube and gas flow system for combined gas-chromatography-atomic emission detection[J]. Anal Chem, 1990, 62: 1027-1034. [36] PONTES F V M, CARNEIRO M, VAITSMAN D S, MONTEIRO M I C, NETO A A, TRISTO M L B. Investigation of the Grignard reaction and experimental conditions for the determination of inorganic mercury and methylmercury in crude oils by GC-ICP-MS[J]. Fuel, 2014, 116: 421-426. [37] SCHICKLING C, BROEKAERT J A C. Determination of mercury species in gas condensates by on line coupled high performance liquid chromatography and cold vapor atomic absorption spectrometry[J]. Appl Organomet Chem, 1995, 9: 29-36. [38] ZETTLIZER M, SCHOLER H F, EIDEN R, FALTER R. Determination of elemental, inorganic and organic mercury in North German gas condensates and formation brines[C]. International Symposium on Oilfield Chemistry, 1997, Houston, Texas, USA. [39] YUN Z J, HE B, WANG Z H, WANG T, JIANG G N. Evaluation of different extraction procedures for determination of organic mercury species in petroleum by high performance liquid chromatography coupled with cold vapor atomic fluorescence spectrometry[J]. Talanta, 2013, 106: 60-65. [40] WILHELM S M, BLOOM N. Mercury in petroleum[J]. Fuel Process Technol, 2000, 63: 1-27. [41] YAN T Y. Mercury removal from oils[J]. Chem Eng Commun, 2000, 177: 15-29. [42] 蒋斌, 蒋洪, 张磊.凝析油脱汞工艺方案研究[J].现代化工, 2018, 38(4): 201-205.JIANG Bin, JIANG Hong, ZHANG Lei. A study on processes of mercury removal from natural gas condensate[J]. Modern Chem Ind, 2018, 38(4): 201-205. [43] 林富荣, 曾天亮.天然气脱汞吸附剂的制备及其性能评价[J].石油与天然气化工, 2019, 48(1): 38-44.LIN Fu-rong, ZENG Tian-liang. Preparation and performance evaluation of mercury removal adsorbent for natural gas[J]. Chem Eng Oil Gas, 2019, 48(1): 38-44. [44] CANDELON J C, PUCCI A, JUBIN C. Process for elimination of mercury contained in a hydrocarbon feed with hydrogen recycling[P]. US Patent No. 9011676, 2013-08-29. [45] SURESH KUMAR REDD K, AL SHOAIBI A, SRINIVASAKANNAN C. Gas-phase mercury removal through sulfur impregnated porous carbon[J]. J Ind Eng Chem, 2014, 20: 2969-2974. [46] ZHANG H, SUN H, ZHANG D, ZHANG W, CHEN S, LI M, LIANG P. Nanoconfinement of Ag nanoparticles inside mesoporous channels of MCM-41 molecule sieve as a regenerable and H2O resistance sorbent for Hg0 removal in natural gas[J]. Chem Eng J, 2019, 361: 139-147. [47] CHALKIDIS A, JAMPAIAH D, HARTLEY P G, SABRI Y M, BHARGAVA S K. Mercury in natural gas streams: A review of materials and processes for abatement and remediation[J]. J Hazard Mater, 2020, 382: 121036. [48] KHAIRI N A S, YUSOF N A, ABDULLAH A H, MOHAMMAD F. Removal of toxic mercury from petroleum oil by newly synthesized molecularly-imprinted polymer[J]. Int J Mol Sci, 2015, 16: 10562-10577. [49] NASIRIMOGHADDAM N S, ZEINAIL S, SABBAGHI S. Chitosan coated magnetic nanoparticles as nano-adsorbent for efficient removal of mercury contents from industrial aqueous and oily samples[J]. J Ind Eng Chem, 2015, 27: 79-87. -





 下载:
下载: