Study on the performance of F-T component modified KCuZrO2 catalyst for CO hydrogenation to isobutanol
-
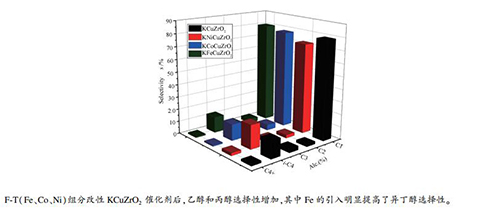
摘要: 采用共沉淀法分别制备了不同F-T组分(Fe、Co、Ni)改性的KCuZrO2催化剂,并用于催化CO加氢合成异丁醇。通过BET、XRD、TEM、XPS、H2-TPR、CO-TPD以及in-situ DRIFTS对催化剂进行了表征。结果显示,F-T组分的加入促进了乙醇和丙醇的形成,但是对异丁醇选择性影响不同。结果表明,Fe促进了催化剂中各组分的分散,活性组分Cu在催化剂表面发生了富集,提高了H2/CO活化吸附;另外,KFeCuZrO2的催化剂表面含有较多的C1物种,有利于乙醇和丙醇进一步发生β-加成反应得到异丁醇,而Co和Ni改性的催化剂上缺少足够的C1物种,因此,异丁醇的选择性并未明显增加。Co的引入对催化剂结构以及Cu的分散影响不大,但是Co改性后催化剂性能有所下降,其原因是催化剂发生了失活;Ni添加后催化剂比表面积有所减小,且催化剂表面Cu/Zr物质的量比也降低到0.19,催化剂粒径增大,Cu-Zr之间相互作用减弱,异丁醇选择性降低。Abstract: KCuZrO2 catalysts modified with different F-T elements (Fe, Co, Ni) were prepared by co-precipitation method and then were tested for isobutanol synthesis from catalytic CO hydrogenation. The catalysts were characterized by N2 adsorption and desorption experiments (BET), XRD, TEM, XPS, H2-TPR, CO-TPD and in situ DRIFTS. The results showed that the addition of F-T components promoted the formation of ethanol and propanol, and had different effects on the selectivity of isobutanol. The characterization results showed that Fe promoted the dispersion of catalyst components, and enriched the active component Cu on the catalyst surface, which improved the active adsorption of H2 and CO. In addition, more C1 species were formed on the KFeCuZrO2 catalyst surface, and these C1 species could further react with ethanol and propanol to produce isobutanol. However, the catalysts modified by Co and Ni lacked sufficient C1 species, so the selectivity of isobutanol did not increase significantly. The introduction of Co had little effect on the structure of catalyst and the dispersion of Cu, but the activity of catalyst decreased after Co addition, which might be due to the deactivation of catalyst. After adding Ni to the catalyst, the specific surface area decreased and the particle size increased, and the Cu/Zr molar ratio on the catalyst surface also decreased to 0.19. The interaction between Cu-Zr was weakened, and the isobutanol selectivity was also reduced.
-
Key words:
- CO hydrogenation /
- F-T elements /
- Cu/ZrO2 /
- isobutanol
-
表 1 KMCuZrO2催化剂的织构性质
Table 1 Textural properties of the fresh KMCuZrO2 catalysts (M: Fe, Co, Ni)
Catalyst ABET/(m2·g-1) vPN/(cm3·g-1) d/nm KCuZrO2 74.12 0.088 4.76 KFeCuZrO2 102.95 0.128 4.97 KCoCuZrO2 88.26 0.126 5.71 KNiCuZrO2 72.38 0.147 8.16 表 2 KMCuZrO2催化剂的XPS表征
Table 2 XPS results of KMCuZrO2 catalysts (M: Fe, Co, Ni)
Catalyst Binding energy E/eV Relative surface concentration of catalysts /% Cu/Zr
(molar ratio)Oads /% Cu 2p3/2 Zr 3d5/2 Cu Zr F-T O K KCuZrO2 933.85 181.61 4.40 14.80 - 72.13 8.67 0.29 22.36 KFeCuZrO2 933.75 181.48 6.54 19.12 - 68.88 5.46 0.34 40.22 KCoCuZrO2 932.94 181.59 5.56 18.83 0.47 69.11 6.03 0.29 50.60 KNiCuZrO2 932.73 181.63 3.79 20.16 0.49 69.30 6.26 0.19 46.02 表 3 KMCuZrO2催化剂上CO吸附位点的分布
Table 3 Distribution of CO adsorption sites over KMCuZrO2 catalysts (M: Fe, Co, Ni)
Catalyst α/(α+β) β/(α+β) KCuZrO2 77.46 22.54 KFeCuZrO2 73.40 26.60 KCoCuZrO2 78.98 21.02 KNiCuZrO2 71.28 28.72 表 4 KMCuZrO2催化剂上CO加氢反应的性能
Table 4 Performance of KMCuZrO2 catalysts for CO hydrogenation (M: Fe, Co, Ni)
Catalyst CO conv.
x/%C-balance
wmol/%Alc. STY/
(g·L-1·h-1)Selectivity sC-atom/% Alc. distribution w/% alc. CHx CO2 DME C1 C2 C3 i-C4 C4+ KCuZrO2 39.30 107 187.04 42.47 26.62 30.90 0.01 78.91 2.16 3.21 14.06 1.66 KFeCuZrO2 52.11 107 210.02 37.21 27.52 35.26 0.01 72.10 2.54 3.93 19.25 2.18 KCoCuZrO2 29.94 106 122.11 37.57 29.47 32.94 0.02 77.71 4.41 3.75 13.13 1.00 KNiCuZrO2 39.55 105 199.23 48.26 22.70 29.03 0.01 79.98 3.09 3.14 12.70 1.09 reaction conditions: p=10.0 MPa, GHSV=3000 h-1, t=360 ℃, H2/CO=2:1, K(%)=4.5, nF-T/nCu=0.040, nCu/nZr=1:3 -
[1] 李明阳.合成气制异丁醇的催化剂研究[D].上海: 华东理工大学, 2015.LI Ming-yang. Study on the catalyst for isobutanol synthesis from syngas[D]. Shanghai: East China University of Science and Technology, 2015. [2] SLATING T A, KESAN J P. Making regulator innovation keep pace with technological innovation[J]. Wis Law Rev, 2011, 1109-1179. http://papers.ssrn.com/sol3/papers.cfm?abstract_id=1805008 [3] 田宇, 王义强, 王启业.异丁醇生物合成的研究进展[J].生物技术通报, 2013, 1(5):40-44. http://d.old.wanfangdata.com.cn/Periodical/swjstb201305007TIAN Yu, WANG Yi-qiang, WANG Qi-ye. Research progress of isobutanol biosynthesis[J]. Biotechnol Bull, 2013, 1(5):40-44. http://d.old.wanfangdata.com.cn/Periodical/swjstb201305007 [4] 寇永利, 解红娟, 刘广波, 张欣悦, 韩怡卓, Noritatsu Tsubaki, 谭猗生. ZnCr基催化剂煅烧温度对异丁醇合成性能的影响[J].燃料化学学报, 2013, 41(6):703-709. http://www.ccspublishing.org.cn/article/id/100032923KOU Yong-li, XIE Hong-juan, LIU Guang-bo, ZHANG Xin-yue, HAN Yi-zhuo, NORITATSU Tsubaki, TAN Yi-sheng. Effect of calcination temperature on the performance of ZnCr based catalyst in isobutanol synthesis[J]. J Fuel Chem Technol, 2013, 41(6):703-709. http://www.ccspublishing.org.cn/article/id/100032923 [5] GAO X F, WU Y Q, ZHANG T, WANG L Y, LI X L, TAN Y S. Binary ZnO/Zn-Cr nanospinel catalysts prepared by a hydrothermal method for isobutanol synthesis from syngas[J]. Cat Sci Technol, 2018, 8:2975-2986. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=226b723dcf76346db9b42eba5a2c714b [6] KOU J W, CHENG S Y, GAO Z H, CHENG F Q, HUANG W. Synergistic effects of potassium promoter and carbon fibers on direct synthesis of isobutanol from syngas over Cu/ZnO/Al2O3 catalysts obtained from hydrotalcite-like compounds[J]. Solid State Sci, 2019, 87:138-145. doi: 10.1016/j.solidstatesciences.2018.11.007 [7] 谭理, 武应全, 张涛, 解红娟, 陈建刚.沉淀温度对K-CuLaZrO2催化剂上合成气直接合成异丁醇的影响[J].燃料化学学报, 2019, 47(9):1096-1102. http://www.ccspublishing.org.cn/article/id/2fda3d52-f899-42f6-bc1f-9e4ac30af8a9TAN Li, WU Ying-quan, ZHANG Tao, XIE Hong-juan, CHEN Jian-gang. Effect of precipitation temperature on the performance of K-CuLaZrO2 catalyst for isobutanol synthesis from syngas[J]. J Fuel Chem Technol, 2019, 47(9):1096-1102. http://www.ccspublishing.org.cn/article/id/2fda3d52-f899-42f6-bc1f-9e4ac30af8a9 [8] 何代平.二氧化锆基催化剂上合成低碳醇和酮的研究[D].北京: 中国科学院大学, 2004.HE Dai-ping. Higher alcohol and ketone synthesis study on ZrO2-based catalysts[D]. Beijing: University of Chinese Academy of Science, 2004. [9] ARTYUKH Y N, LUNEY N K, VERKHGRADSKII O P, ZELENKOV G A, OEVA L A, LIMOVICH E A. Effect of the introduction of certain additives to the CO+H2 mixture and the scheme for the formation of alcohols higher than C1[J]. Theor Exp Chem, 1990, 26:476-479. doi: 10.1007/BF00530266 [10] BERETTA A, LIETTI L, TRONCONI E, FORZATTI P, PASQUON I. Development of a mechanistic kinetic model of the higher alcohol synthesis over a Cs-doped Zn/Cr/O catalyst 2. Analysis of chemical enrichment experiments[J]. Ind Eng Chem Res, 1996, 35:2154-2160. http://cn.bing.com/academic/profile?id=1e02bcc2e1fa846d77613980e5ce6f37&encoded=0&v=paper_preview&mkt=zh-cn [11] WU Y Q, XIE H J, KOU Y L, NORITASTU T, HAN Y Z, TAN Y S. The mechanism of higher alcohol formation on ZrO2-based catalyst from syngas[J]. Korean J Chem Eng, 2015, 32:406-412. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=09529b8232223a919a67b18e74c6f8cb [12] XU X, DOESBURG E B M, SCHOLTEN J J F. Synthesis of higher alcohols from syngas recent patented catalysts and tentative on the mechanism[J]. Catal Today, 1987, 2:125-170. doi: 10.1016/0920-5861(87)80002-0 [13] AO M, PHAM G H, SUNARSO J, TADE M O, LIU S. Active centers of catalysts for higher alcohol synthesis from syngas:A review[J]. ACS Catal, 2018, 8:7025-7050. http://cn.bing.com/academic/profile?id=05159d839423c235a4a24f794f0c8f0d&encoded=0&v=paper_preview&mkt=zh-cn [14] LUK H T, MONDELI C, FERRE D C, STEWART J A, PEREZ-RAMIREZ J. Status and prospects in higher alcohols synthesis from syngas[J]. Chem Soc Rev, 2017, 46:1358-1426. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=cb9b83963a9d190538889e1e6504dd9f [15] WANG P, CHEN S Y, BAI Y X, GAO X F, LI X L, SUN K, XIE H J, YANG G H, HAN Y Z, TAN Y S. Effect of the promoter and support on cobalt-based catalysts for higher alcohols synthesis through CO hydrogenation[J]. Fuel, 2017, 195:69-81. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=8e0383f680d04082ad7dbc676b216ca4 [16] SPIVEY J J, EGBEBI A. Heterogeneous catalytic synthesis of ethanol from biomass-derived syngas[J]. Chem Soc Rev, 2007, 36:1514-1528. http://cn.bing.com/academic/profile?id=7ef6fbd5fc9f8ff0c4a525e4bed428ed&encoded=0&v=paper_preview&mkt=zh-cn [17] XIAO K, BAO Z, QI X, WANG X, ZHONG L, FANG K, LIN M, SUN Y. Advances in bifunctional catalysis for higher alcohol synthesis from syngas[J]. Chin J Catal, 2013, 34:116-129. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=cuihuaxb201301010 [18] XIAO K, QI X, BAO Z, WANG X, ZHONG L, FANG K, LIN M, SUN Y. CuFe, CuCo and CuNi nanoparticles as catalysts for higher alcohol synthesis from syngas:a comparative study[J]. Catal Sci Technol, 2013, 3:1591-1602. http://cn.bing.com/academic/profile?id=f9e7c76f3ed9ed8a0f2ffb8293ec622c&encoded=0&v=paper_preview&mkt=zh-cn [19] SUN X, YU Y, ZHANG M. Insight into the effect of promoter Co on C2 oxygenate formation from syngas on CoCu(100) and Cu(100):a comparative DFT study[J]. Appl Surf Sci, 2018, 434:28-39. doi: 10.1016/j.apsusc.2017.10.164 [20] AO M, PHAM G H, SAGE V, PAREEK V. Selectivity enhancement for higher alcohols synthesis product in Fischer-Tropsch synthesis over nickel-substituted La0.9Sr0.1CoO3 perovskite catalysts[J]. Fuel, 2017, 206:390-400. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=7dd22d52b5bd2d0ca3cc88393c35ed59 [21] AO M, PHAM G H, SAGE V, PAREEK V, LIU S M. Perovskite-derived trimetallic Co-Ni-Cu catalyst for higher alcohol synthesis from syngas[J]. Fuel Process Technol, 2019, 193:141-148. http://cn.bing.com/academic/profile?id=eeed1464c5ec4e18522192fbdf918ac1&encoded=0&v=paper_preview&mkt=zh-cn [22] 陈小平. F-T合成组元改性Cu/Mn/ZrO2甲醇催化剂用于低碳醇合成的研究[D].北京: 中国科学院大学, 1999.CHEN Xiao-ping. Study on F-T element modified Cu/Mn/ZrO2 methanol catalysts for higher alcohol synthesis[D]. Beijing: University of Chinese Academy of Science, 1999. [23] XU R, YANG C, WEI W, LI W H, SUN Y H, HU T D. Influence of promoter on catalytic properties of Cu-Mn-Fe/ZrO2 catalysts for alcohols synthesis[J]. React Kinet Catal Lett, 2004, 81:91-98. https://www.researchgate.net/publication/229307965_Fe-modified_CuMnZrO2_catalysts_for_higher_alcohols_synthesis_from_syngas [24] LI W Z, HUANG H, LI H J, ZHANG W, LIU H C. Facile synthesis of pure monoclinic and tetragonal zirconia nanoparticles and their phase effects on the behavior of supported molybdena catalysts for methanol-selective oxidation[J]. Langmuir, 2008, 24:8358-8366. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=cee0b513bef781546d5210b8717e74d8 [25] LI G R, LI W, ZHANG M H, TAO K Y. Characterization and catalytic application of homogeneous nano-composite oxides ZrO2-Al2O3[J]. Catal Today, 2004, 93(5):95-601. doi: 10.1016/j.cattod.2004.06.010 [26] SUN K, WU Y Q, TAN M H, WANG L Y, YANG G H, ZHANG M, ZHANG W, TAN YS. Ethanol and higher alcohols synthesis from syngas over CuCoM (M=Fe, Cr, Ga and Al) nanoplates derived from hydrotalcite-like precursors[J]. ChemCatChem, 2019, 11:2695-2706. doi: 10.1002/cctc.201900096 [27] AVGOUROPOULOS G, IOANNIDES T, MATRALIS H. Influence of the preparation method on the performance of CuO-CeO2 catalysts for the selective oxidation of CO[J]. Appl Catal B:Environ, 2005, 56:87-93. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=3887d474fb479182a25d534d11f5c3ba [28] WU Y Q, XIE H J, TIAN S P, NORITATSU T, HAN Y Z, TAN Y S. Isobutanol synthesis from syngas over K-Cu/ZrO2-La2O3(x) catalysts:Effect of La-loading[J]. J Mol Catal A:Chem, 2015, 396:254-260. doi: 10.1016/j.molcata.2014.10.003 [29] YU Q, YAO X J, ZHANG H L, GAO F, DONG L. Effect of ZrO2 addition method on the activity of Al2O3-supported CuO for NO reduction with CO:Impregnation vs. co-precipitation[J]. Appl Catal A:Gen, 2012, 423/424:42-51. doi: 10.1016/j.apcata.2012.02.017 [30] RIBEIRO N F P, SOUZA M M V M, SCHMAL M. Combustion synthesis of copper catalysts for selective CO oxidation[J]. J Power Sources, 2008, 179:329-330. doi: 10.1016/j.jpowsour.2007.12.096 [31] WU Y Q, WANG S C, XIE H J, GAO J W, TIAN S P, HAN Y Z, TAN Y S. Influence of Cu on the K-LaZrO2 Catalyst for Isobutanol Synthesis[J]. Acta Phys Chim Sin, 2015, 31(1):166-172. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=wlhxxb201501027 [32] JUNG K T, BELL A T. An in situ infrared study of dimethyl carbonate synthesis from carbon dioxide and methanol over zirconia[J]. J Catal, 2001, 204:339-347. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=7474d7a365d9e184f2579bb6375b8233 [33] POKROVSKI K, JUNG K T, BELL A T. Investigation of CO and CO2 adsorption on tetragonal and monoclinic zirconia[J]. Langmuir, 2001, 17:4297-4300. doi: 10.1016/j.jpowsour.2007.12.096 [34] SANTOS V P, VANDER L B, CHOJECKI A, BUDRONI G, CORTHALS S, SHIBATA H, MEIMA G R, KAPTEIJIN F, MAKKEE M, GASCON J. Mechanistic insight into the synthesis of higher alcohols from syngas:The role of K promotion on MoS2 catalysts[J]. ACS Catal. 2013, 3:1634-1637. doi: 10.1021/cs4003518 [35] WU Y Q, GONG N N, ZHANG M, ZHANG W, ZHANG T, ZHANG J F, WANG L Y, XIE H J, TAN Y S. Insight into the branched alcohol formation mechanism on K-ZnCr catalysts from syngas[J]. Catal Sci Technol, 2019, 9(10):2592-2600. doi: 10.1039/C9CY00542K -





 下载:
下载: