Effect of TiO2 doping on methane catalytic combustion deoxidation of CuMnCe/Al2O3 catalyst
-
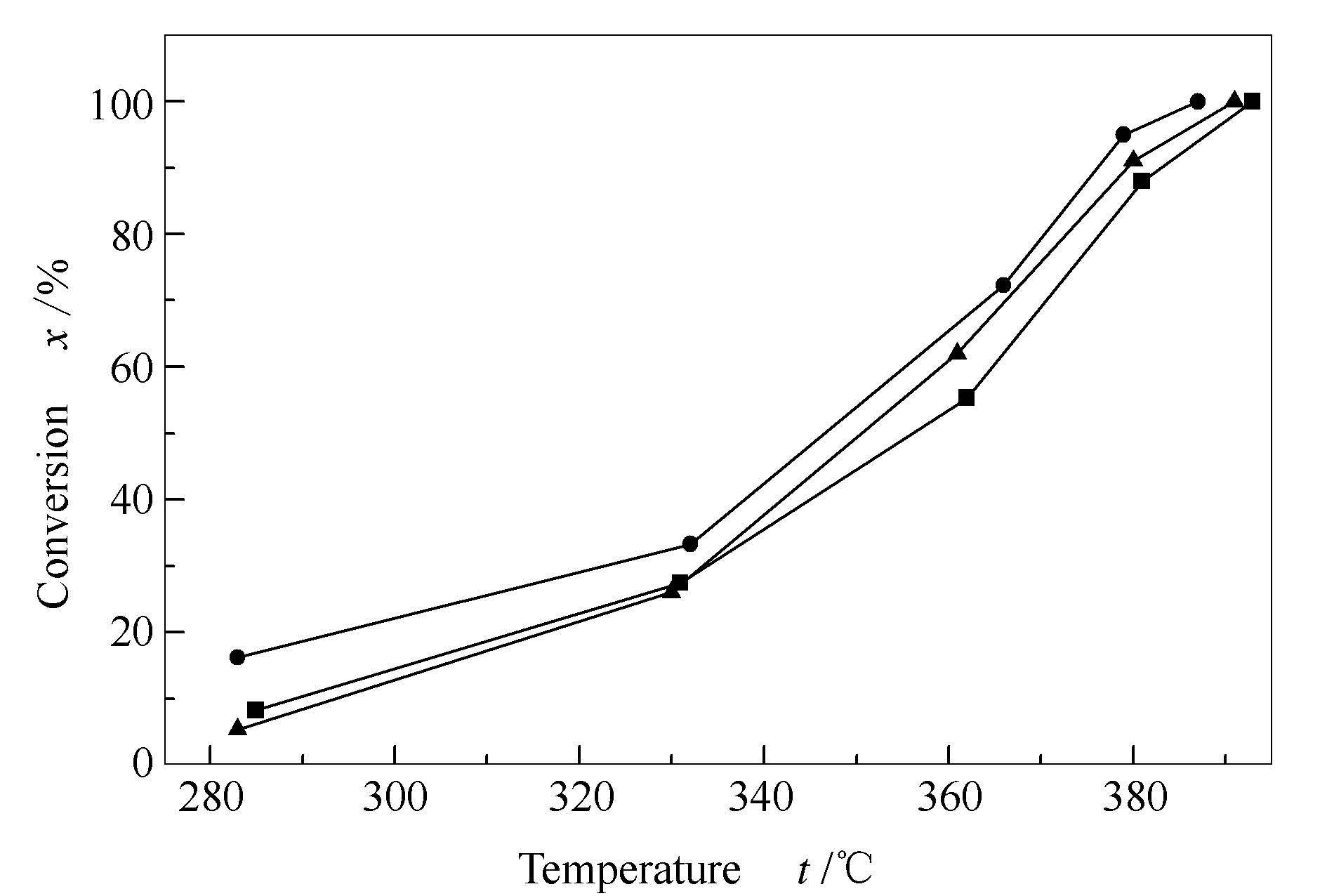
摘要: 以掺杂了不同TiO2含量的Al2O3作为载体,通过等体积浸渍法制备了一系列不同TiO2含量的CuMnCe/TiO2-Al2O3催化剂,用BET、H2-TPR、XRD和XPS表征技术对催化剂物理化学性质进行表征,并考察了催化剂在含甲烷气脱氧反应中的催化性能。结果表明,在载体中添加TiO2对催化剂活性组分的晶相结构和分散度没有明显影响;但有效改善了Al2O3载体抗烧结能力;增加了CuMnCe/Al2O3催化剂表面Ce3+/(Ce3++Ce4+)的相对含量,从而提高了活性氧的移动性,且使催化剂表面可氧化还原物种含量和表面吸附氧Osur/(Osur+Olatt)的含量增多。有效改善了催化剂在含甲烷气催化燃烧脱氧上的催化活性。其中,CuMnCe/4% TiO2-Al2O3表现出最优的催化活性,在387℃时可使含甲烷气中氧气的转化率达到100%。Abstract: CuMnCe/TiO2-Al2O3 catalysts with different TiO2 contents were prepared by the co-impregnation method and characterized by BET, XRD, XPS and H2-TPR techniques.The catalytic performance in methane deoxidation reaction were investigated using CGK-5A fixed bed reactor.Results showed that doping some TiO2 in the Al2O3 support has no effect on the crystalline structure of the active ingredient.But it can effectively improve Al2O3 support sintering resistance and thermal stability, furthermore, it increases content of Ce3+/(Ce3++Ce4+) in the CuMnCe/Al2O3 catalysts, which improves the mobility of oxygen.Besides, the content of adsorbed oxygen Osur/(Osur+Olatt) and reducible species in the catalyst surface are increased.Thus, effectively, doping some TiO2 into the Al2O3 support improved the catalytic activity of deoxygenation catalyst for methane combustion.CuMnCe/4% TiO2-Al2O3 exhibited optimum catalytic activity and oxygen conversion rate reached 100% at 387℃.
-
Key words:
- titania /
- alumina /
- deoxygenation /
- CuMnCe catalyst /
- adsorbed oxygen
-
表 1 载体的比表面积和孔结构特性
Table 1 Surface area and pore characteristics of different support
Sample Surface area
A/(m2·g-1)Pore volume
v/(mL·g-1)Average pore size
d/nmAl2O3(450 ℃) 199.77 0.442 9 8.87 Al2O3(600 ℃) 172.08 0.459 1 10.67 Al2O3(800 ℃) 144.19 0.456 2 12.56 TiO2-Al2O3 (450 ℃) 163.48 0.404 6 9.89 TiO2-Al2O3 (600 ℃) 151.54 0.412 1 10.87 TiO2-Al2O3 (800 ℃) 125.31 0.405 7 12.95 表 2 催化剂表面Ce3+以及Osur的含量和150-350 ℃ 的H2-TPR氢气消耗峰的面积
Table 2 Ratio of Ce3+and Osur in the catalyst surface by XPS and relative H2 consumption peak area during H2-TPR experiment from 150 ℃ up to 350 ℃
Sample Ce3+/( Ce3++ Ce4+)/%a Osur/(Osur+Olatt)/%a H2 consume(peak areab) CuMnCe/Al2O3 45.14 40.65 3 683 CuMnCe/4%TiO2-Al2O3 46.28 41.95 4 421 CuMnCe/8%TiO2-Al2O3 39.62 42.76 4 216 a: calculated by XPS results; b: calculated by H2-TPR results 表 3 不同催化剂表相中各元素结合能及各组分的浓度
Table 3 Content and binding energy on the surface of different catalysts
Sample Binding energy E/eV Content of catalysts surface w/% Cu 2p3/2 Mn 2p3/2 O 1s Al 2p Cu Mn O Al CuMnCe/Al2O3 933.4 642.2 531.1 74.2 0.24 0.45 52.7 34.3 CuMnCe/4%TiO2-Al2O3 933.2 641.8 531.1 74.2 0.38 0.59 52.1 34.4 CuMnCe/8%TiO2-Al2O3 933.4 642.1 531.1 74.2 0.45 0.80 51.2 35.4 -
[1] 吴剑峰, 孙兆虎, 公茂琼. 从含氧煤层气中安全分离提纯甲烷的工艺方法[J]. 天然气工业, 2009, 29(2):113-116+146.WU Jian-feng, SUN Zhao-hu, GONG Mao-qiong. Security technology of methane recovery from coalbed gas with oxygen[J]. Nat Gas Ind, 2009, 29(2):113-116+146. [2] 聂李红, 徐绍平, 苏艳敏, 刘淑琴. 低浓度煤层气提纯的研究现状[J]. 化工进展, 2008, 27(10):1505-1511+1521.NIE Li-hong, XU Shao-ping, SU Yan-min, LIU Shu-qin. Progress of recovery of low concentration coal bed methane[J]. Chem Ind Eng Prog, 2008, 27(10):1505-1511+1521. [3] 王树东, 袁中山, 王胜, 张纯希, 倪长军. 一种煤层气脱氧催化剂、其制备方法及应用:中国, 101664679B[P]. 2012-09-12.(WANG Shu-dong, YUAN Zhong-shan, WANG Sheng, ZHANG Chun-xi, NI Chang-jun. A preparation method and application of coal bed methane deoxygenation catalyst:CN, 101664679B[P]. 2012-09-12.) [4] 袁善良, 薄其飞, 蒋毅. 催化燃烧法用于煤层气脱氧的研究进展[J]. 天然气化工(C1化学与化工), 2016, 41(5):73-77.YUAN Shan-liang, BO Qi-fei, JIANG Yi. Reasearch advances in coalbed methane (CBM) deoxygeneration by catalytic combustion[J]. Nat Gas Chem Ind, 2016, 41(5):73-77. [5] OH S H, MITCHELL P J. Effects of rhodium addition on methane oxidation behavior of alumina-supported noble metal catalysts[J]. Appl Phys B, 1994, 5(1/2):165-179. [6] BURCH R, LOADER P K. Investigation of Pt/Al2O3 and Pd/Al2O3 catalysts for the combustion of methane at low concentrations[J]. Appl Phys B, 1994, 5(1):149-164. [7] LYUBOVSKY M, PFEFFERLE L. Methane combustion over the α, -alumina supported Pd catalyst:Activity of the mixed Pd/PdO state[J]. Appl Phys A, 1998, 179(1):107-119. [8] HAOJIE G, ZHONGQING Y, LI Z, JINGYU R, YANRONG C. Experimental and kinetic study of methane combustion with water over copper catalyst at low-temperature[J]. Energy Convers Manage, 2015, 103:244-250. doi: 10.1016/j.enconman.2015.06.076 [9] HAOJIE G, ZHONGQING Y, LI Z, JINGYU R, YUNFEI Y, MINGNV G. Effects of O2/CH4 ratio on methane catalytic combustion over Cu/γ, -Al2O3 particles[J]. Int J Hydrogen Energy, 2016, 41(40):18282-18290. doi: 10.1016/j.ijhydene.2016.08.134 [10] 唐国旗, 张春富, 孙长山, 严斌, 杨国祥, 戴伟, 田保亮. 活性氧化铝载体的研究进展[J]. 化工进展, 2011, 30(8):1756-1765.TANG Guo-qi, ZHANG Chun-fu, SUN Chang-shan, YAN Bin, YANG Guo-xiang, DAI Wei, TIAN Bao-liang. Research progress of γ, -alumina support[J]. Chem Ind Eng Prog, 2011, 30(8):1756-1765. [11] MATAM S K, AGUIRRE M H, WEIDENKAFF A, FERRI D. Revisiting the problem of active sites for methane combustion on Pd/Al2O3 by operando XANES in a lab-scale fixed-bed reactor[J]. J Phys Chem C, 2010, 114(20):9439-9443. doi: 10.1021/jp1019697 [12] BRAGA V S, DIAS J A, DIAS S C L, DE MACEDO J L. Catalyst materials based on Nb2O5 supported on SiO2-Al2O3:Preparation and structural characterization[J]. Chem Mater, 2005, 17(3):690-695. doi: 10.1021/cm048673u [13] ESCOBAR J, ANTONIO DE LOS REYES J, VIVEROS T. Nickel on TiO2-modified Al2O3 sol-gel oxides:Effect of synthesis parameters on the supported phase properties[J]. Appl Phys A, 2003, 253(1):151-163. [14] WANG L, HUANG M, LI B, DONG L, JIN G, GAO J, MA J, LIU T. Enhanced hydrothermal stability and oxygen storage capacity of La3+ doped CeO2-γ, -Al2O3 intergrowth mixed oxides[J]. Ceram Int, 2015, 41(10, Part A):12988-12995. doi: 10.1016/j.ceramint.2015.06.142 [15] RODSEANGLUNG T, RATANA T, PHONGAKSORN M, TUNGKAMANIA S. Effect of TiO2 Incorporated with Al2O3 on the hydrodeoxygenation and hydrodenitrogenation CoMo sulfide catalysts[J]. Energy Procedia, 2015, 79:378-384. doi: 10.1016/j.egypro.2015.11.506 [16] LARTIGUE KORINEK S, LEGROS C, CARRY C, HERBST F. Titanium effect on phase transformation and sintering behavior of transition alumina[J]. J Eur Ceram Soc, 2006, 26(12):2219-2230. doi: 10.1016/j.jeurceramsoc.2005.04.006 [17] REDDY B M, KHAN A, YAMADA Y, KOBAYASHI T, LORIDANT S, VOLTA J C. Structural characterization of CeO2-TiO2 and V2O5/CeO2-TiO2 catalysts by Raman and XPS techniques[J]. J Phys Chem B, 2003, 107(22):5162-5167. doi: 10.1021/jp0344601 [18] WANG H, CHEN X, GAO S, WU Z, LIU Y, WENG X. Deactivation mechanism of Ce/TiO2 selective catalytic reduction catalysts by the loading of sodium and calcium salts[J]. Catal Sci Technol, 2013, 3(3):715-722. doi: 10.1039/C2CY20568H [19] LIOTTA L F, DI CARLO G, PANTALEO G, VENEZIA A M, DEGANELLO G. Co3O4/CeO2 composite oxides for methane emissions abatement:Relationship between Co3O4-CeO2 interaction and catalytic activity[J]. Appl Phys B, 2006, 66(3/4):217-227. [20] LU H, KONG X, HUANG H, ZHOU Y, CHEN Y. Cu-Mn-Ce ternary mixed-oxide catalysts for catalytic combustion of toluene[J]. J Environ Sci, 2015, 32:102-107. doi: 10.1016/j.jes.2014.11.015 [21] TANG X, XU Y, SHEN W. Promoting effect of copper on the catalytic activity of MnOx, -CeO2 mixed oxide for complete oxidation of benzene[J]. Chem Eng J, 2008, 144(2):175-180. doi: 10.1016/j.cej.2008.01.016 -





 下载:
下载: