Constructions of coal and char molecular models based on the molecular simulation technology
-
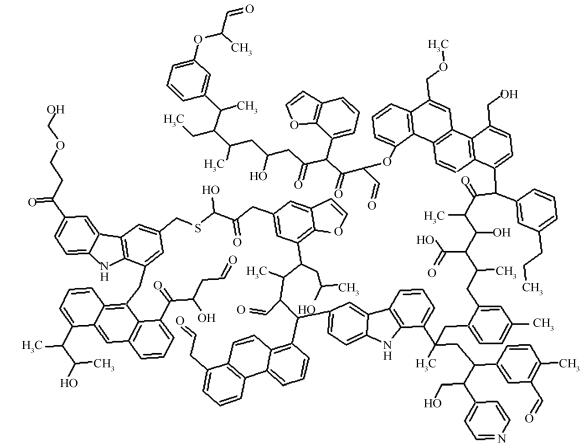
摘要: 煤、焦是过程工业的重要原料。因此,有必要深入了解煤、焦分子结构以揭示其反应性。采用Materials Studio 7.0软件,从分子层次研究煤、焦的分子结构。根据已报道的文献,构建煤、焦的初始结构;基于分子力学原理对这些结构进行优化,使得模型物性与煤、焦物性相符;基于退火模拟算法对模型进行优化,从而使得模型密度、元素分析数据与真实值吻合;基于能量最小化原理,对煤、焦模型再次优化,从而获得其稳定、真实的分子构型。由计算结果发现,模型的估算密度、元素组成与已报道一致,说明构建的模型是有效、合理的;在模型优化过程中,相对于其他能量而言,库伦能和范德华能起着重要的作用。因此可以推断在煤、焦热加工过程中,弱键占据主要地位。另外,本文采用分子模拟技术构建煤、焦模型的方法对于构建其他复杂大分子结构有着重要的借鉴作用。Abstract: Coal and char are essential energy sources for the process industry. Insightful understanding of those molecules is useful to explore reactivities of coal and char. Therefore, coal and char molecular structures were investigated at atomic level using Materials Studio 7.0 software. Firstly, coal and char initial structures were constructed based on reported literatures. Secondly, those structures were improved by molecular mechanics, where functional group fragments were added to satisfy the property of coal or char. Then, the subsequent structures were optimized by annealing dynamics simulation to adjust density and elementary composition. Finally, the potential energies of coal and char were calculated using energy minimization method. It was pointed out that the estimated densities and elementary composition were agreements with the published literatures, which indicated that those structures were valid and reasonable. From the simulated results, it was shown that the Coulomb energy and van der Waals energy played a much more important role than other energies during the stabilizing molecular construction process. Thus, it was inferred that the weak bond was predominant in the thermal processing of coal or char. In addition, this work demonstrated that the molecular simulation technology was meaningful to construct the complex macromolecular structure.
-
Key words:
- coal char /
- molecular simulation /
- molecular dynamics /
- annealing dynamics simulation
-
Table 2 Molecule composition of coal molecular model

Table 3 Elements analysis of simulated coal and char

-
[1] World Coal Association, Annual Energy Report[EB/OL]. http://www.worldcoal.org/coal/, (accessed 2011). [2] China Analysis Report. U. S. Energy Information Administration (EIA)[EB/OL]. http://www.eia.gov/countries/cab.cfm?fips=CH, (accessed March 28, 2013). [3] FLETCHER T H, SOLUM M S, GRANT D M, PUGMIRE R J. Chemical structure of char in the transition from devolatilization to combustion[J]. Energy Fuels, 1992, 6(5):643-650. doi: 10.1021/ef00035a016 [4] YU J L, LUCAS J A, WALL T F. Formation of the structure of chars during devolatilization of pulverized coal and its thermoproperties:A review[J]. Prog Energy Combust Sci, 2007, 33(2):135-170. doi: 10.1016/j.pecs.2006.07.003 [5] ZENG D, FLETCHER T H. Effects of pressure on coal pyrolysis and char morphology[J]. Energy Fuels, 2005, 19(5):1828-1838. doi: 10.1021/ef0500078 [6] LU L M, SAHAJWALLA V, HARRIS D. Characteristics of chars prepared from various pulverized coals at different temperatures using drop-tube furnace[J]. Energy Fuels, 2000, 14(4):869-876. doi: 10.1021/ef990236s [7] YOSHIZAWA N, MARUYAMAMA K, YAMASHITA T, AKIMOTO A. Dependence of microscopic structure and swelling property of DTF chars upon heat-treatment temperature[J]. Fuel, 2006, 85(14):2064-2070. http://www.sciencedirect.com/science/article/pii/S0016236106001190 [8] KIDENA K, MATSUMOTO K, KATSUYAMA M, MURATA S, NOMURA M. Development of aromatic ring size in bituminous coals during heat treatment in the plastic temperature range[J]. Fuel Process Technol, 2004, 85(8):827-835. http://www.sciencedirect.com/science/article/pii/S0378382003002881 [9] KULAOTS I, HSU A, SUUBERG E M. The role of porosity in char combustion[J]. Proc Combust Inst, 2007, 31(2):1897-1903. doi: 10.1016/j.proci.2006.08.004 [10] MATSUOKA K, AKAHANE T, ASO H, SHARMA A, TOMITA A. The size of polyaromatic layer of coal char estimated from elemental analysis data[J]. Fuel, 2008, 87(4):539-545. http://www.sciencedirect.com/science/article/pii/S0016236107001214 [11] DAVIS K A, HURT R H, YANG N Y C, HEADLEY T J. Evolution of char chemistry, crystallinity, and ultrafine structure during pulverized-coal combustion[J]. Combust Flame, 1995, 100(1):31-40. http://www.sciencedirect.com/science/article/pii/001021809400062W [12] SHENG C. Char structure characterised by Raman spectroscopy and its correlations with combustion reactivity[J]. Fuel, 2007, 86(15):2316-2324. doi: 10.1016/j.fuel.2007.01.029 [13] CAI H Y, GUELL A J, CHATZAKIS I N, LIM J Y, DUGWELL D R, KANDIYOTI R. Combustion reactivity and morphological change in coal chars:Effect of pyrolysis temperature, heating rate and pressure[J]. Fuel, 1996, 75(1):15-24. doi: 10.1016/0016-2361(94)00192-8 [14] HURT R H. Reactivity distributions and extinction phenomena in coal char combustion[J]. Energy Fuels, 1993, 7(6):721-733. doi: 10.1021/ef00042a005 [15] LIU G S, NIKSA S. Coal conversion submodels for design applications at elevated pressures. Part Ⅱ. Char gasification[J]. Prog Energy Combust Sci, 2004, 30(6):679-717. doi: 10.1016/j.pecs.2004.08.001 [16] SOLOMON P R, SERIO M A, SUUBERG E M. Coal pyrolysis:Experiments, kinetic rates and mechanisms[J]. Prog Energy Combust Sci, 1992, 18(2):133-220. doi: 10.1016/0360-1285(92)90021-R [17] RADOVIC L R, WALKER P L, JENKINS R G. Importance of catalyst dispersion in the gasification of lignite chars[J]. J Catal, 1983, 82(2):382-394. doi: 10.1016/0021-9517(83)90205-1 [18] DOMAZETIS G, JAMES B D, LIESEGANG J. Computer molecular models of low-rank coal and char containing inorganic complexes[J]. J Mol Model, 2008, 14(7):581-597. doi: 10.1007/s00894-008-0309-9 [19] DOMAZETIS G, JAMES B D, LIESEGANG J. High-level computer molecular modeling for low-rank coal containing metal complexes and iron-catalyzed steam gasification[J]. Energy Fuels, 2008, 22(6):3994-4005. doi: 10.1021/ef800457t [20] DOMAZETIS G, LIESEGANG J, JAMES B D. Studies of inorganics added to low-rank coals for catalytic gasification[J]. Fuel Process Technol, 2005, 86(5):463-486. doi: 10.1016/j.fuproc.2004.03.009 [21] ZHANG Y, CHEN D Y, ZHANG D, ZHU X F. TG-FTIR analysis of bio-oil and its pyrolysis/gasification property[J]. J Fuel Chem Technol, 2012, 40(10):1194-1199. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18043.shtml [22] XU X Q, WANG Y G, CHEN Z D, BAI L, ZHANG K J, YANG S S, ZHANG S. Influence of cooling treatments on char microstructure and reactivity of Shengli brown coal[J]. J Fuel Chem Technol, 2015, 43(1):1-8. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18547.shtml [23] MATHEWS J P, van Duin A, CHAFFEE A. The utility of coal molecular models[J]. Fuel Process Technol, 2011, 92(4):718-728. doi: 10.1016/j.fuproc.2010.05.037 [24] CASTRO-MARCANO F, KAMAT A M, RUSSO JR M F, van Duin A C T, MATHEWS J P. Combustion of an Illinois No.6 coal char simulated using an atomistic char representation and the ReaxFF reactive force field[J]. Combust Flame, 2012, 159:1272-1285. doi: 10.1016/j.combustflame.2011.10.022 [25] STUART S J, TUTEIN A B, HARRISON J A. A reactive potential for hydrocarbons with intermolecular interactions[J]. J Chem Phys, 2000, 112(14):6472-6486. doi: 10.1063/1.481208 [26] VAN DUIN A C T, DASGUPTA S, LORANT F, GODDARD W A. ReaxFF:A reactive force field for hydrocarbons[J]. J Phys Chem A, 2001, 105(41):9396-9409. doi: 10.1021/jp004368u [27] HUANG L P, KIEFFER J. Molecular dynamics study of cristobalite silica using a charge transfer three-body potential:Phase transformation and structural disorder[J]. J Chem Phys, 2003, 118(3):1487-1498. doi: 10.1063/1.1529684 [28] VAN DUIN A C T, STRACHAN A, STEWMAN S, ZHANG Q S, XU X, GODDARD W A. ReaxFFSiO reactive force field for silicon and silicon oxide systems[J]. J Phys Chem A, 2003, 107(19):3803-3811. doi: 10.1021/jp0276303 [29] VIOLI A. Modeling of soot particle inception in aromatic and aliphatic premixed flames[J]. Combust Flame, 2004, 139(4):279-287. doi: 10.1016/j.combustflame.2004.08.013 [30] CHENOWETH K, VAN DUIN A C T, GODDARD W A. ReaxFF reactive force field for molecular dynamics simulations of hydrocarbon oxidation[J]. J Phys Chem A, 2008, 112(5):1040-1053. doi: 10.1021/jp709896w [31] ROGEL E, CARBOGNANI L. Density estimation of asphaltenes using molecular dynamics simulations[J]. Energy Fuels, 2003, 17(2):378-86. doi: 10.1021/ef020200r [32] TAKANOHASHI T, KAWASHIMA H. Construction of a model structure for upper freeport coal using 13C NMR chemical shift calculations[J]. Energy Fuels, 2002, 16:379-387. doi: 10.1021/ef0101154 [33] Materials Studio Help, 2007[K]. Accelrys Software Inc. , San Diego, USA. [34] LEI Z, YANG B, WEI J. Improved inheritance algorithm for the assembly of coal fragments[J]. Ind Eng Chem Res, 2015, 50:12392-12399. doi: 10.1021/ie201715q [35] CHEN H, LI J, LEI Z, GE L. Microwave-assisted extraction of shenfu coal and its macromolecule structure[J]. Min Sci Technol, 2009, 19(1):19-24. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=zhkd200901006&dbname=CJFD&dbcode=CJFQ [36] ZHENG M, LI X, LIU J, GUO L. Initial chemical reaction simulation of coal pyrolysis via ReaxFF molecular dynamics[J]. Energy Fuels, 2013, 27:2942-2951. doi: 10.1021/ef400143z [37] NOSÉ S. A unified formulation of the constant temperature molecular dynamics methods[J]. J Chem Phys, 1984, 81:511-519. doi: 10.1063/1.447334 [38] ZHENG M, LI X, LIU J, WANG Z, GONG X, GUO L, SONG W. Pyrolysis of Liulin coal simulated by GPU-Based ReaxFF MD with chemin for matics analysis[J]. Energy Fuels, 2014, 28:522-534. doi: 10.1021/ef402140n [39] CHEREPANOV V B, CYR S L M, SOUTHERN B W. Metastable states of the potts glass[J]. J Phys A:Math Gen, 1992, 25(16):4347. doi: 10.1088/0305-4470/25/16/012 -





 下载:
下载: