Preparation of compound magnetic Fe3O4/MgAl-layered double hydroxide and its application in biodiesel transesterification
-
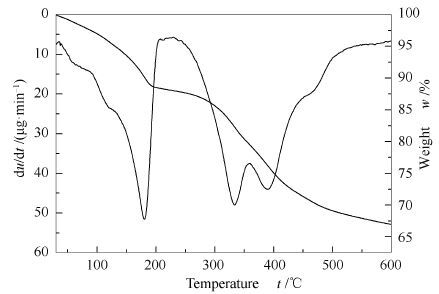
摘要: 使用共沉淀法制备磁性Fe3O4粒子,并以此为原料制备出Fe3O4/MgAl-LDH,将其焙烧产物Fe3O4/Mg(Al)O作为微藻油脂和甲醇发生酯交换反应的催化剂,利用产物生物柴油产率作为评价催化剂的活性指标,探究其酯交换活性。通过TG-DTG、XRD、SEM、EDS、TEM、N2吸附-脱附、VSM等表征手段对所制备样品进行表征。结果表明,MgAl-LDH、Mg(Al)O在Fe3O4表面生长,并具有一定的磁响应能力。在反应时间为4 h、醇油物质的量比为13:1的条件下,生物柴油产率高达90%。经过三次使用后,仍具有一定活性。在外磁场的作用下可完成催化剂与反应物的分离。Abstract: Magnetic Fe3O4 was synthesized by chemical coprecipitation method, and used as a raw material for preparing Fe3O4/MgAl-LDH. The calcined Fe3O4/Mg(Al)O was taken as the catalyst for the transesterification of microalgae oil with methanol, and the biodiesel yield was used to evaluate the activity of the catalyst. Several instruments including TG-DTG, XRD, SEM, EDS, TEM, N2 absorption-desorption and VSM were employed to characterize the catalysts. The results show that MgAl-LDH and Mg(Al)O are successfully formed on Fe3O4 particles which have a strong magnetic response. When the reaction time is 4 h and the methanol/oil mol ratio is 13:1, the biodiesel oil yield reaches to 90%. After using for three times the catalyst still has certain activity and can be easily separated and recycled.
-
Key words:
- biodiesel /
- magnetism /
- transesterification /
- hydrotalcite
-
表 1 微藻油脂的理化性质
Table 1 Physical and chemical properties of microalgae oil
Number Acid value w/(mgKOH·g-1) Saponification value w/(mgKOH·g-1) Moisture and volatile matter x/% Average molecular weight q/(g·mol-1) 1 0.93 188.346 0.18 898.00 2 0.93 188.137 0.18 899.00 3 0.94 188.570 0.17 896.00 Average value 0.93 188.351 0.18 898.00 表 2 不同样品的比表面积和孔结构
Table 2 Results of N2 absorption-desorption measurements for different samples
Catalyst BET surface area A/(m2·g-1) Total pore volume v/(cm3·g-1) Pore size d/nm Mg(Al)O 138 0.62 11.15 Fe3O4/ Mg(Al)O 228 0.92 13.50 -
[1] CREASEY J J, CHIEREGATO A, MANAYIL J C, PARLETT C M, WILSON K, LEE A F.Alkali-and nitrate-free synthesis of highly active Mg-Al hydrotalcite-coated alumina for FAME production[J].Catal Sci Technol, 2014, 4(3):861-870. doi: 10.1039/C3CY00902E [2] LIN C Y, CHENG H H.Application of mesoporous catalysts over palm-oil biodiesel for adjusting fuel properties[J].Energy Convers Manage, 2012, 53(1):128-134. doi: 10.1016/j.enconman.2011.08.019 [3] MACOR A, PAVANELLO P.Performance and emissions of biodiesel in a boiler for residential heating[J].Energy, 2009, 34(12):2025-2032. doi: 10.1016/j.energy.2008.08.021 [4] DENG X, FANG Z, LIU Y.Ultrasonic transesterification of Jatrophacurcas L.oil to biodiesel by a two-step process[J].Energy Convers Manage, 2010, 51(12):2802-2807. doi: 10.1016/j.enconman.2010.06.017 [5] 文利柏, 谭文广, 王运, 韩鹤友, 郑新生.固体酸催化剂催化乌桕籽油制备生物柴油[J].中国油脂, 2008, 33(6):44-47. http://www.cnki.com.cn/Article/CJFDTOTAL-ZYZZ200806013.htmWEN Li-bai, TAN Wen-guang, WANG Yun, HAN He-you, ZHENG Xin-sheng.Biodiesel preparation from stillingia oil by solid acid catalyzing[J].China Oils Fats, 2008, 33(6):44-47. http://www.cnki.com.cn/Article/CJFDTOTAL-ZYZZ200806013.htm [6] 岳鹍, 王兴国, 金青哲, 刘元法.固体酸催化剂酸催化生物柴油合成反应性能的比较[J].中国油脂, 2006, 31(7):63-65. http://www.cnki.com.cn/Article/CJFDTOTAL-ZYZZ200607019.htmYUE Kun, WANG Xing-guo, JIN Qing-zhe, LIU Yuan-fa.Synthesis of biodiesel from frying oil catalyzed by solid acid catalyst[J].China Oils Fats, 2006, 31(7):63-65. http://www.cnki.com.cn/Article/CJFDTOTAL-ZYZZ200607019.htm [7] PULLEN J, SAEED K.Investigation of the factors affecting the progress of base-catalyzed transesterification of rapeseed oil to biodiesel FAME[J].Fuel Process Technol, 2015, 130:127-135. doi: 10.1016/j.fuproc.2014.09.013 [8] SHIBASAKI-KITAKAWA N, HONDA H, KURIBAYASHi H, TODA T, FUKUMURA T, YONEMOTO T.Biodiesel production using anionic ion-exchange resin as heterogeneous catalyst[J].Bioresource Technol, 2007, 98:416-421. doi: 10.1016/j.biortech.2005.12.010 [9] LIEN Y S, HSIEH L S, WU J C S.Biodiesel synthesis by simultaneous esterificationand transesterification using oleophilic acid ctalyst[J].Ind Eng Chem Res, 2010, 49(5):2118-2121. doi: 10.1021/ie901496h [10] LOTERO E, LIU Y, LOPEZ D E, SUWANNAKARN K, BRUCE D A, GOODWIN J G.Synthesis of biodiesel via acid catalysis[J].Ind Eng Chem Res, 2005, 44(14):5353-5363. doi: 10.1021/ie049157g [11] 王建黎, 李永超, 徐之超, 计建炳.超声波辐射对醇油不相容体系酯交换反应的影响[J].中国油脂, 2006, 31(4):61-63. http://www.cnki.com.cn/Article/CJFDTOTAL-ZYZZ200604020.htmWANG Jian-li, LI Yong-chao, XU Zhi-chao, JI Jian-bing.Effect of ultrasonic on the transesterification of methanol-oil immiscible system[J].China Oils Fats, 2006, 31(4):61-63. http://www.cnki.com.cn/Article/CJFDTOTAL-ZYZZ200604020.htm [12] SREE R, KURIAKOSE S.Alkali salts of heteropoly tungstates:Efficient catalysts for the synthesis of biodiesel from edible and non-edible oils[J].J Energy Chem, 2015, 24(1):87-92. doi: 10.1016/S2095-4956(15)60288-1 [13] 赵策, 曾虹燕, 黄炎, 刘平乐, 王亚举, 杨永杰, 张伟.镁铁水滑石的制备及其对小球藻油脂合成生物柴油的催化性能[J].燃料化学学报, 2012, 40(3):337-344. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract17906.shtmlZHAO Ce, ZENG Hong-yan, HUANG Yan, LIU Le-ping, WANG Ya-ju, YANG Yong-jie, ZHANG Wei.Preparation of MgFe-hydrotalcites and their catalytic performance in synthesis of biodiesel oil from chlorella protothecoides oil[J].J Fuel Chem Technol, 2012, 40(3):337-344. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract17906.shtml [14] 张慧, 徐彦红, 段雪.磁性纳米固体碱催化剂MgAl-OH-LDHs/NiFe2O4的合成、表征与性能研究[J].化学学报, 2004, 62(8):750-756.ZHANG Hui, XU Yan-hong, DUAN Xue.Synthesis characterization and properties of a nano-scale magnetic solid base catalyst MgAl-OH-LDHs/NiFe2O4[J].J Chem Ind Eng, 2004, 62(8):750-756. [15] 盘登科, 张慧.核壳结构磁性镁铝复合氧化物亚微米粒子的制备与表征[J].无机化学学报, 2011, 27(7):1341-1347. http://www.cnki.com.cn/Article/CJFDTOTAL-WJHX201107021.htmPAN Deng-ke, ZHANG Hui.Synthesis and characterization of magnetic Mg-Al mixed oxides submicro particles with core-shell structure[J].Chin J Inorg Chem, 2011, 27(7):1341-1347. http://www.cnki.com.cn/Article/CJFDTOTAL-WJHX201107021.htm [16] TRUJILLANO R, HOLGADO M J, PIGAZO F, RIVES V.Preparation physicochemical characterisation and magnetic properties of Cu-Al layered double hydroxides with CO32- and anionic surfactants with different alkyl chains in the interlayer[J].Phys B, 2006, 373(2):267-273. doi: 10.1016/j.physb.2005.11.154 [17] SHAO M, NING F, ZHAO J, WEI M, EVANS D G, DUAN X.Preparation of Fe3O4@SiO2@layered double hydroxide core-shell microspheres for magnetic separation of proteins[J].J Am Chem Soc, 2012, 134(2):1071-1077. doi: 10.1021/ja2086323 [18] LIU W, ZHONG W, DU Y W.Magnetic nanoparticles with core/shell structures[J].J Nanosci Nanotechnol, 2008, 8(6):2781-2792. [19] AY A N, ZVMREOGLU-KARAN B, TEMEL A, RIVES V.Bioinorganic magnetic core-shell nanocomposites carrying antiarthritic agents:Intercalation of ibuprofen and glucuronic acid into Mg-Al-layered double hydroxides supported on magnesium ferrite[J].Inorg Chem, 2009, 48(18):8871-8877. doi: 10.1021/ic901097a [20] CHEN D, LI Y, ZHANG J, ZHOU J Z, GUO Y, LIU H.Magnetic Fe3O4/ZnCr-layered double hydroxide composite with enhanced adsorption and photocatalytic activity[J].Chem Eng J, 2012, 185:120-126. [21] HU S, GUAN Y, WANG Y, HAN H.Nano-magnetic catalyst KF/CaO-Fe3O4 for biodiesel production[J].Appl Energy, 2011, 88(8):2685-2690. doi: 10.1016/j.apenergy.2011.02.012 [22] XUE B J, LUO J, ZHANG F, FANG Z.Biodiesel production from soybean and Jatropha oils by magnetic CaFe2O4-CaFe2O5-based catalyst[J].Energy, 2014, 68:584-591. doi: 10.1016/j.energy.2014.02.082 [23] TANG S, WANG L, ZHANG Y, LI S, TIAN S, WANG B.Study on preparation of Ca/Al/Fe3O4 magnetic composite solid catalyst and its application in biodiesel transesterification[J].Fuel Process Technol, 2012, 95:84-89. doi: 10.1016/j.fuproc.2011.11.022 [24] LEI X, ZHANG F, YANG L, GUO X, TIAN Y, FU S, DUAN X.Highly crystalline activated layered double hydroxides as solid acid-base catalysts[J].AIChE J, 2007, 53(4):932-940. doi: 10.1002/(ISSN)1547-5905 [25] 阎杰, 丘泰球.甘油铜比色法测定甘油含量的研究[J].中国油脂, 2004, 29(1):40-43. http://www.cnki.com.cn/Article/CJFDTOTAL-ZYZZ200401010.htmYAN Jie, QIU Tai-qiu, Determination of glycerol by cupric glycerinate colorimetry[J].China Oils Fats, 2004, 29(1):40-43. http://www.cnki.com.cn/Article/CJFDTOTAL-ZYZZ200401010.htm [26] CASENAVE S, MARTINEZ H, GUIMON C, AUROUX A, HULEA V, DUMITRIU E.Acid-base properties of MgCuAl mixed oxides[J].J Therm Anal Calorim, 2003, 72(1):191-198. doi: 10.1023/A:1023980005672 [27] 刘秀芳, 范彬彬, 高升成, 李瑞丰.C/MgAl水滑石复合材料为前驱物所制催化剂在柠檬醛丙酮缩合反应中的催化性能[J].无机化学学报, 2013, 29(2):345-349.LIU Xiu-fang, FAN Bin-bin, Gao Sheng-cheng, LI Rui-feng.Citral-acetone condensation over catalysts prepared from C/MgAl hydrotalcite hybrids[J].Chin J Inorg Chem, 2013, 29(2):345-349. [28] SHOU J, JIANG C, WANG F, QIU M, XU Q.Fabrication of Fe3O4 MgAl-layered double hydroxide magnetic composites for the effective decontamination of Co (Ⅱ) from synthetic wastewater[J].J Mol Liq, 2015, 207:216-223. doi: 10.1016/j.molliq.2015.03.047 [29] GURSKY J A, BLOUGH S D, LUNA C, GOMEZ C, LUEVANO A N, GARDNER E A.Particle-particle interactions between layered double hydroxide nanoparticles[J].J Am Chem Soc, 2006, 128(26):8376-8377. doi: 10.1021/ja0612100 -





 下载:
下载: