Crystal transformation synthesis, hydrogenation activity and sulfur-tolerant performance of Pt particles encapsulated in sodalite
-
摘要: 通过晶体转化的方法合成封装Pt的方钠石Pt/ SOD。 封装Pt的方钠石通过以下两步结晶的方法合成,在100℃结晶12h,120℃结晶144h,130℃结晶96h,140℃结晶60h,150℃结晶42h160℃结晶30h。样品在硫化氢的毒化前后表现了良好的加氢活性。通过X射线衍射法检测样品相的结晶形式。通过H2-TPD表征给体相Pt/ SOD与受体相HZS-5之间的氢溢流。Abstract: Noble metals are widely used as hydrogenation catalysts for refining and modifying fuel oil. However, their stability is still a problem. Thus, crystal transformation method was used here to encapsulate platinum (Pt) particles in sodalite through two steps. First, the sample was crystallized at 100 ℃ for 12 h. Then, it was further crystallized at 120 ℃ for 144 h, 130 ℃ for 96 h, 140 ℃ for 60 h, 150 ℃ for 42 h, or 160 ℃ for 30 h. The resultant solid (designated as Pt/SOD) shows high activity and excellent sulfur-tolerant performance in benzene hydrogenation. The crystalline phases were identified by X-ray diffraction technique. The hydrogen spillover effect of Pt/SOD and the spillover hydrogen acceptability of HZSM-5 were investigated with H2-TPD.
-
Key words:
- synthesis /
- encapsulation /
- sodalite /
- crystal transformation /
- sulfur tolerance
-
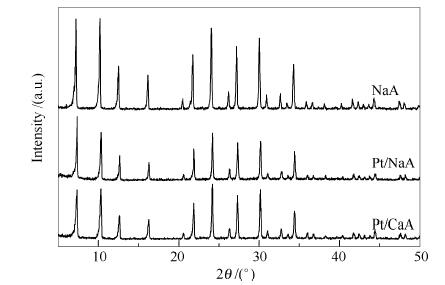
Table 1 Benzene conversions obtained over different catalysts
Entry Sample Conversion x/%a 1 Pt/NaA 0 2 Pt/CaA 14.81 3 Pt/NaA+HZSM-5 58.01 4 Pt/NaA-SP+HZSM-5 0 a: determined by GC using the area normalization method Table 2 Benzene conversions obtained over Pt/SOD-M and its physical mixture with HZSM-5 (M represents the crystallization temperature)
Sample Conversion x/%a without HZSM-5 mixed with HZSM-5 mixed with HZSM-5 after being poisoned by sulfur Pt/SOD-120 0 26.69 15.29 Pt/SOD-130 0 35.65 30.90 Pt/SOD-140 0 40.81 32.33 Pt/SOD-150 0 45.17 43.28 Pt/SOD-160 0 3.74 3.51 a: determined by GC using the area normalization method -
[1] BENSERADJ F, SADI F, CHATER M. Hydrogen spillover studies on diluted Rh/Al2O3 catalyst[J]. Appl Catal A: Gen, 2002, 228(11): 135-144. [2] CECKIEWICZ S, DELMON B. Cooperative action of Pt/γ-Al2O3 catalyst and γ-Al2O3 diluent in the hydrogenation of benzene[J]. J Catal, 1987, 108(2): 294-303. doi: 10.1016/0021-9517(87)90179-5 [3] CHEN H, YANG H, OMOTOSO O, DING L, BRIKER Y, ZHENG Y, RING Z. Contribution of hydrogen spillover to the hydrogenation of naphthalene over diluted Pt/RHO catalysts[J]. Appl Catal A: Gen, 2009, 358(2): 103-109. doi: 10.1016/j.apcata.2008.12.045 [4] CHEN S, CHEN J, GIELECIAK R, FAIRBRIDGE C. Reactivity characteristics of Pt-encapsulated zeolite catalysts for hydrogenation and hydrodesulfurization[J]. Appl Catal A: Gen, 2012, 415-416: 70-79. doi: 10.1016/j.apcata.2011.12.005 [5] CHOI M, WU Z, IGLESIA E. Mercaptosilane-assisted synthesis of metal clusters within zeolites and catalytic consequences of encapsulation[J]. J Am Chem Soc, 2010, 132(26): 9129-9137. doi: 10.1021/ja102778e [6] FLANIGEN E M, BENNETT J M, GROSE R W, COHEN J P, PATTON R L, KIRCHNER R M. Silicalite, a new hydrophobic crystalline silica molecular sieve[J]. Nature, 1978, 271: 512-516. doi: 10.1038/271512a0 [7] GOEL S, WU Z, ZONES S I, IGLESIA E. Synthesis and catalytic properties of metal clusters encapsulated within small-pore (SOD, GIS, ANA) zeolites[J]. J Am Chem Soc, 2012, 134(42): 17688-17695. doi: 10.1021/ja307370z [8] CONNER W C, FALCONER J L. Spillover in heterogeneous catalysis[J]. Chem Rev, 1995, 95: 759-788. doi: 10.1021/cr00035a014 [9] GOTTI A, PRINS R. Basic metal oxides as co-catalysts in the conversion of synthesis gas to methanol on supported palladium catalysts[J]. J Catal, 1998, 175(2): 302-311. doi: 10.1006/jcat.1998.1996 [10] GREER H, WHEATLEY P S, ASHBROOK S E, MORRIS R E, ZHOU W. Early stage reversed crystal growth of zeolite A and its phase transformation to sodalite[J]. J Am Chem Soc, 2009, 131(49): 17986-17992. doi: 10.1021/ja907475z [11] GUCZI L, KIRICSI I. Zeolite supported mono-and bimetallic systems: Structure and performance as CO hydrogenation catalysts[J]. Appl Catal A: Gen, 1999, 186(1/2): 375-394. https://www.researchgate.net/publication/222981794_Zeolite_supported_mono-and_bimetallic_systems_Structure_and_performance_as_CO_hydrogenation_catalysts [12] KHOOBIAR S. Particle to particle migration of hydrogen atoms on platinum-alumina catalysts from particle to neighboring particles[J]. J Phys Chem, 1964, 68(2): 411-412. doi: 10.1021/j100784a503 [13] OHGOSHI S, NAKAMURA I, WAKUSHIMA Y. Hydrogenation of isobutylene by spillover hydrogen from Pt/KA-zeolite to NaY-zeolite[J]. Stud Surf Sci Catal, 1993, 77: 289-292. doi: 10.1016/S0167-2991(08)63193-6 [14] REAGAN W J, CHESTER A W, KERR G T. Studies of the thermal decomposition and catalytic properties of some platinum and palladium ammine zeolites[J]. J Catal, 1981, 69(1): 89-100. doi: 10.1016/0021-9517(81)90131-7 [15] REED T B, BRECK D W. Crystalline zeolites.Ⅱ: Crystal structure of synthetic zeolite, type A[J]. J Am Chem Soc, 1956, 78(23): 5972-5977. doi: 10.1021/ja01604a002 [16] SACHTLER W M H. Metal clusters in zeolites: An intriguing class of catalysts[J]. Chem Res,1993, 26(7): 383-387. doi: 10.1021/ar00031a005 [17] SINFELT J H, LUCCHESI P J. Kinetic evidence for the migration of reactive intermediates in surface catalysis[J]. J Am Chem Soc, 1963, 85(21): 3365-3367. doi: 10.1021/ja00904a012 [18] SONG G, LIU H, LI F, LV Z, XUE J. Synthesis and characterization of LTA zeolite with uniform intracrystal mesopores directed by bridged polysilsesquioxane monomer[J]. J Porous Mater, 2014, 21(6): 1101-1111. doi: 10.1007/s10934-014-9860-1 [19] SRINIVAS S T, RAO P K. Direct observation of hydrogen spillover on carbon-supported platinum and its influence on the hydrogenation of benzene[J]. J Catal, 1994, 148(2): 470-477. doi: 10.1006/jcat.1994.1233 [20] WEISE P B, FRILETTE V J, MOWER E B, MAATMAN R W. Catalysis by crystalline aliminosilicates Ⅱ: Molecular-shape selective reactions[J]. J Catal, 1962, 1(4): 307-312. doi: 10.1016/0021-9517(62)90058-1 [21] YANG H, CHEN H, CHEN J, OMOTOSO O, RING Z. Shape selective and hydrogen spillover approach in the design of sulfur-tolerant hydrogenation catalysts[J]. J Catal, 2006, 243(1): 36-42. doi: 10.1016/j.jcat.2006.06.026 [22] YANG H, CHEN H, DU H, HAWKINS R, CRAIG F, RING Z, OMOTOSO O, MUNOZ V, MIKULA R. Incorporating platinum precursors into a NaA-zeolite synthesis mixture promoting the formation of nanosized zeolite[J]. Microprous Mesprous Mater, 2009, 117(1): 33-40. https://www.researchgate.net/profile/Honglin_Chen/publication/222017186_Incorporating_platinum_precursors_into_a_NaA-zeolite_synthesis_mixture_promoting_the_formation_of_nanosized_zeolite/links/00b7d537d4932dfb5d000000.pdf?origin=publication_list [23] ZHAN B Z, IGLESIA E. RuO2 clusters within LTA zeolite cages: Consequences of encapsulation on catalytic reactivity and selectivity[J]. Chem Int Ed, 2007, 46(20): 3697-3700. doi: 10.1002/(ISSN)1521-3773 [24] ZHAN B Z, WHITE M A, SHAM T K, PINCOCK J A, DOUCET R J, RAMANA RAO K V, ROBERTSON K N, CAMERON T S. Zeolite-Confined Nano-RuO2: A green, selective, and efficient catalyst for aerobic alcohol oxidation[J]. J Am Chem Soc, 2003, 125(8): 2195-2199. doi: 10.1021/ja0282691 [25] ZLOTEA C, CAMPESI R, CUEVAS F, LEROY E, DIBANDJO P, VOLKRINGER C, LOISEAU T, FEREY G, LATROCHE M. Size-dependent hydrogen sorption in ultrasmall Pd clusters embedded in a mesoporous carbon template[J]. J Am Chem Soc, 2010, 132(22): 2991-2997. https://www.researchgate.net/publication/44594759_Size-Dependent_Hydrogen_Sorption_in_Ultrasmall_Pd_Clusters_Embedded_in_a_Mesoporous_Carbon_Template [26] ZHIJIE W, SARIKA G, MINKEE C, ENRIQUE I. Hydrothermal synthesis of LTA-encapsulated metal clusters and consequences for catalyst stability, reactivity, and selectivity[J]. J Catal, 2014, 311: 458-468. doi: 10.1016/j.jcat.2013.12.021 [27] SONGHYUN L, KYUNGHO L, JUHWAN I, HYUNGJUN K, MINKEE C. Revisiting hydrogen spillover in Pt/LTA: Effects of physical diluents having different acid site distributions[J]. J Catal, 2015, 325: 26-34. doi: 10.1016/j.jcat.2015.02.018 -





 下载:
下载: