Mechanism for the enhancement of the resistance of the OMS-2 mercury oxidation catalyst to sulfur by modifying with cerium
-
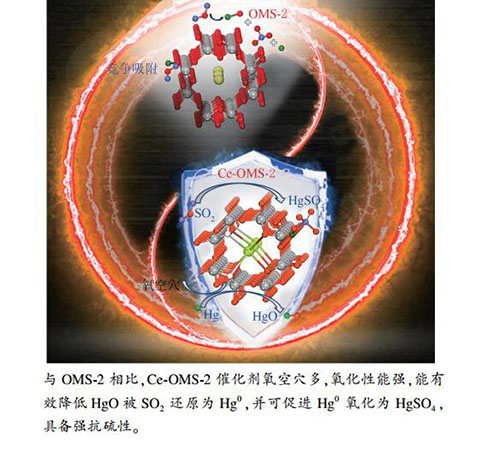
摘要: 针对氧化锰八面体分子筛(OMS-2)在催化氧化单质汞过程中抗硫性能差的问题,采用铈(Ce)对OMS-2催化剂进行改性提高其抗硫性。通过热力学分析、固定床实验、氮吸附、XRD、ICP和XPS等表征手段,研究了铈增强OMS-2抗硫能力的原因。结果表明,Ce改性得到的催化剂比表面积大、空隙结构丰富,可以在催化剂表面通过化学吸附吸附更多的Hg0;Ce改性得到的催化剂会造成Mn缺陷生成,提高电子迁移率,使得吸附氧(Oβ)占比高,可提供更多的活性位点;OMS-2催化剂表面氧化的HgO可经不同浓度的SO2还原为Hg0或者HgSO4,但Ce改性得到的催化剂可使该部分还原产物迅速重新氧化成HgO,提高了表观Hg0氧化效率。该研究结果可为开发高性能抗硫汞氧化催化剂提供理论依据。Abstract: In view of the poor resistance of manganese oxide octahedral molecular sieve (OMS-2) catalyst to sulfur in the oxidation of elemental mercury, CeO2 was used to modify the OMS-2 catalyst. The mechanism for the enhancement of the resistance of the OMS-2 catalyst to sulfur by modifying with CeO2 was investigated, with the help of thermodynamic analysis, fixed-bed reaction test and various characterization methods like nitrogen sorption, XRD ICP and XPS. The results indicate: The OMS-2 catalyst modified by Ce has a large surface area and void-rich structure, which can adsorb more Hg0 through chemical adsorption; More Mn defects are formed on the OMS-2 catalyst through modifying with Ce, leading to an increase in the electron mobility, the proportion of adsorbed oxygen (Oβ) species, and the density of catalytically active sites; The Ce-modified OMS-2 catalyst can quickly re-oxidize the reduced Hg0 or HgSO4 species (facilely formed on the pristine OMS-2 catalyst surface in the presence of SO2) to HgO, which can then improve the apparent Hg0 oxidation efficiency. The results should be helpful for the development of high-performance anti-thio catalysts for mercury oxidation.
-
Key words:
- mercury oxide /
- SO2 /
- reduction /
- thermodynamics /
- coal-fired flue gas
-
表 1 实验条件
Table 1 Reaction test conditions
Test Catalyst Carrier gas Temp. t/℃ Flow /(L·min-1) 1 OMS-2 N2,N2+0.05%SO2,N2+0.1%SO2,N2+0.1%SO2+4%O2 150 1 2 Ce-OMS-2 N2,N2+0.05%SO2,N2+0.1%SO2,N2+0.1%SO2+4%O2 150 1 3 Ce-OMS-2#1 N2 100-700 0.5 Ce-OMS-2#2 4 OMS-2* N2+0.05%SO2 150 1 5 Ce-OMS-2* N2+0.05%SO2 150 1 6 10 mg HgO N2+0.05%SO2 150 1 note:“#1”, the catalyst was pretreated under N2+4%O2+Hg0 for 1 h;
“#2”, the catalyst was pretreated under N2+4%O2+0.05%SO2+Hg0 for 1 h;
“*”, the catalyst pretreated under N2+4%O2+500 μg/m3 Hg0 for 7 d表 2 催化剂的比表面积和表面原子含量
Table 2 Surface area and surface atomic concentration of the catalysts
Catalyst Surface area A/(m2·g-1) Surface atom concentration by ICP by XPS K/Mn Ce/Mn Mn3+/Mn4+ Ce3+/Ce4+ Oα Oβ OMS-2 173 0.10 - 1.07 - 79.6% 20.4% Ce-OMS-2 390 0.038 0.35 1.10 0.24 68.2% 31.8% note: Oα: lattice oxygen; Oβ: surface adsorbed oxygen 表 3 HgO在SO2气氛下的初始工况
Table 3 Initial conditions of HgO under SO2 atmosphere
Species HgO SO2 O2 SO3 Hg HgSO4 Amount of substance /kmol 1 1 0 0 0 0 -
[1] 王立刚, 彭苏萍, 陈昌和.燃煤飞灰对锅炉烟道气中Hg0的吸附特性[J].环境科学, 2003, 24(6): 59-62.WANG Li-gang, PENG Su-ping, CHEN Chang-he. The experimental study to Hg0 adsorption of fly ash in flue gas[J]. Environ Sci, 2003, 24(6): 59-62. [2] 任建莉, 周劲松, 骆仲泱, 徐璋, 张雪梅.钙基类吸附剂脱除烟气中气态汞的试验研究[J].燃料化学学报, 2006, 34(5): 557-561.REN Jian-li, ZHOU Jin-song, LUO Zhong-yang, XU Zhang, ZHANG Xue-mei. Ca-based sorbents for mercury vapor removal from flue gas[J]. J Fuel Chem Technol, 2006, 34(5): 557-561. [3] ZHAO Y X, MICHAEL D M, EDWIN S O, JOHN H P, GRANT E D. Effects of sulfur dioxide and nitric oxide on mercury oxidation and reduction under homogeneous conditions[J]. J Air Waste Manage Assoc, 2012, 56(5): 628-635. [4] WANG F, WANG H M, ZHANG F, ZHU J W, TIAN G, LIU Y, MAO J X. SO2/Hg removal from flue gas by dry FGD[J]. Int J Min Sci Technol, 2012, 22(1): 107-110. [5] WANG Y Y, SHEN B X, HE C, YUE S J, WANG F M. Simultaneous removal of NO and Hg0 from flue gas over Mn-Ce/Ti-PILCs[J]. Environ Sci Technol, 2015, 49(15): 9355-9363. [6] YANG W, LIU Y X, WANG Q, PAN J F. Removal of elemental mercury from flue gas using wheat straw chars modified by Mn-Ce mixed oxides with ultrasonic-assisted impregnation[J]. Chem Eng J, 2017, 326(15): 169-181. [7] XU H M, QU Z, ZONG X, QUAN F Q, MEI J, YAN N Q. Catalytic oxidation and adsorption of Hg0 over low-temperature NH3-SCR LaMnO3 perovskite oxide from flue gas[J]. Appl Catal B: Environ, 2016, 186(5): 30-40. [8] ATRIBAK I, BUENO L A, GARCIA G A, NAVARO P, FRIAS D, MONTES M. Catalytic activity for soot combustion of birnessite and cryptomelane[J]. Appl Catal B: Environ, 2010, 93(3/4): 267-273. [9] DING Y S, SHEN X F, SITHAMBARAM S, GOMEZ S, KUMAR R, CRISOSTOMO V M B, SUIB S L, AINDOW M. Synthesis and catalytic activity of cryptomelane-type manganese dioxide nanomaterials produced by a novel solvent-free method[J]. Chem Mater, 2005, 17(21): 5382-5389. [10] HUANG W M, SHI J L. Water-promoted low-concentration NO removal at room temperature by Mg-doped manganese oxides OMS-2[J]. Appl Catal A: Gen, 2015, 507(25): 65-74. [11] GANDHE A R, JEANETTE S, REBELLO J, FIGUEIREDO L, FERNANDES J B. Manganese oxide OMS-2 as an effective catalyst for total oxidation of ethyl acetate[J]. Appl Catal B: Environ, 2007, 72(1/2): 129-135. [12] ADJIMI S, GARCIA V J, DIAZ J A, RETAILLEAU L, GIL S, PERA T M, GUO Y, GIROIR F A. Highly efficient and stable Ru/K-OMS-2 catalyst for NO oxidation[J]. Appl Catal B: Environ, 2017, 219(15): 459-466. [13] HERNANDEZ W Y, CENTENO M A, SVETLANA I, PIERRE E, GAIGNEAUXE M, ODRIOZOLA J A. Cu-modified cryptomelane oxide as active catalyst for CO oxidation reactions[J]. Appl Catal B: Environ, 2012, 123(23): 27-35. [14] LI J F, YAN N Q, QU Z, QIAO S H, YANG S J, GUO Y F, LIU P, JIA J P. Catalytic oxidation of elemental mercury over the modified catalyst Mn/alpha-Al2O3 at Lower Temperatures[J]. Environ Sci Technol, 2010, 44(1): 426-431. [15] LIU X, JIANG S J, LI H L, YANG J P, YANG Z Q, ZHAO J X, PENG H Y, KAIMIN S. Elemental mercury oxidation over manganese oxide octahedral molecular sieve catalyst at low flue gas temperature[J]. Chem Eng J, 2019, 356(15): 142-150. [16] WU S J, KATAYAMA R, UDDIN M A, SASAOKA E, XIE Z M. Study on reactivity of HgO over activated carbon with HCl and SO2 in the presence of moisture by temperature-programmed decomposition desorption mass spectrometry[J]. Energy Fuels, 2015, 29(10): 6598-6604. [17] 李扬, 刘冰, 杨赫, 杨大伟, 胡浩权. MnOx-TiO2吸附剂对燃煤烟气中汞的脱除[J].燃料化学学报, 2020, 48(5): 513-524.LI Yang, LIU Bing, YANG He, YANG Da-wei, HU Hao-quan. Removal of elemental mercury(Hg0) from simulated flue gas over MnOx-TiO2 sorbents[J]. J Fuel Chem Technol, 2020, 48(5): 513-524. [18] WU S J, UDDIN M A, NAGANO S, MASAKI O, SASAOKA E. Fundamental study on decomposition characteristics of mercury compounds over solid powder by temperature-programmed decomposition desorption mass spectrometry[J]. Energy Fuels, 2011, 25(2): 144-153. [19] HE S, ZHOU J S, ZHU Y Q, LUO Z Y, NI M J, CEN K. Mercury oxidation over a vanadia-based selective catalytic reduction catalyst[J]. Energy Fuels, 2009, 23(1): 253-259. [20] 赵莉, 何青松, 刘宇, 吴洋文, 陆强, 刘松涛, 董长青.湿法脱硫浆液中Hg2+的还原机制研究[J].燃料化学学报, 2017, 45(6): 755-760.ZHAO Li, HE Qing-song, LIU Yu, WU Yang-wen, LU Qiang, LIU Song-tao, DONG Chang-qing. Mechanism of Hg2+ reduction in wet flue gas desulfurization liquors[J]. J Fuel Chem Technol, 2017, 45(6): 755-760. [21] LI H L, WU C Y, LI Y, ZHANG J. CeO2-TiO2 catalysts for catalytic oxidation of elemental mercury in low-rank coal combustion flue gas[J]. Environ Sci Technol, 2011, 45(17): 7394-400. [22] WANG T, LIU J, YANG Y H, SUI Z F, ZHANG Y S, WANG J W, PAN W P. Catalytic conversion of mercury over Ce doped Mn/SAPO-34 catalyst: Sulphur tolerance and SO2/SO3 conversion[J]. J Hazard Mater, 2020, 381(5): 120986. [23] 王艳坤. HSC Chemistry软件在高校化学科研中的应用[J].河南教育学院学报:自然科学版, 2013, 22(2): 32-34.WANG Yan-kun. Application of HSC chemistry software in university chemical scientific research[J]. J Henan Ins Edu Nat Sci Ed, 2013, 22(2): 32-34. [24] GRANITE E J, PENNLINE H W, HARGIS R A. Novel sorbents for mercury removal from flue gas[J]. Ind Eng Chem Res, 2000, 39(4): 1020-1029. [25] WU Z B, JIANG B Q, YUE L, ZHAO W R, GUAN B H. Experimental study on a low-temperature SCR catalyst based on MnOx/TiO2 prepared by sol-gel method[J]. J Hazard Mater, 2007, 145(3): 488-494. [26] LI H L, ZHU L, WANG J, LI L Q, SHIH K. Development of nano-sulfide sorbent for efficient removal of elemental mercury from coal combustion fuel gas[J]. Environ Sci Technol, 2016, 50(17): 9551-9557. [27] HOU J T, LI Y Z, MAO M Y, ZHAO X J, YUE Y Z. The effect of Ce ion substituted OMS-2 nanostructure in catalytic activity for benzene oxidation[J]. Nanoscale, 2014, 6(24): 15048-15058. [28] LIU C, CHEN L, LI J, MA L, ARANDIYAN H, DU Y, XU J, HAO J. Enhancement of activity and sulfur resistance of CeO2 supported on TiO2-SiO2 for the selective catalytic reduction of NO by NH3[J]. Environ Sci Technol, 2012, 46(11): 6182-6189. [29] WANG X Y, LAN Z X, ZHANG K, CHEN J J, JIANG L L, WANG R H. Structure activity relationships of AMn2O4 (A=Cu and Co) spinels in selective catalytic reduction of NOx: Experimental and theoretical study[J]. J Chem Phys C, 2017, 121(6): 3339-3349. [30] KIVELSON, DANIEL. The determination of the potential constants of SO2 from centrifugal distortion effects[J]. J Chem Phys, 1954, 22(5): 904-908. [31] 王鹏鹰.铝基SCR催化剂催化氧化烟气中汞的实验与机理研究[D].武汉: 华中科技大学, 2014.WANG Peng-ying. A dissertation submitted in partial fulfllment of the requirements for the degree of doctor of philosophy in engineering[D]. Wuhan: Huazhong University of Science and Technology, 2014. [32] 张旭楠. CeO2改性SCR催化剂同时脱除烟气中Hg0和NO的实验研究[D].长沙: 湖南大学, 2015.ZHANG Xu-nan. The experimental study on the simultaneous removal of elemental mercury (Hg0) and NO from flue gas by CeO2 modified SCR catalysts[D]. Changsha: Hunan University, 2015. [33] ZHUANG Y, JASON L, RICHARD L, MIKE H, JOHN P. Impacts of acid gases on mercury oxidation across SCR catalyst[J]. Fuel Process Technol, 2007, 88(10): 929-934. [34] LAUDAL D L, THOMPSON J S, PAVLISH J H, BRICKET L, CHU P, SRIVASTAVA R K, LEE C W, KILGROE J. Mercury speciation at power plants using SCR and SNCR control technologies[J]. Electron Manager, 2012, 53(1): 16-22. [35] SUI Z F, ZHANG Y S, LI W H, WILLIAM O, CAO Y, PAN W P. Partitioning effect of mercury content and speciation in gypsum slurry as a function of time[J]. J Therm Anal Calorim, 2015, 119(3): 1611-1618. [36] ZHANG Y S, ZHAO L L, GUO R T, WANG J W, CAO Y, WILLIAM O, PAN W P. Influences of NO on mercury adsorption characteristics for HBr modified fly ash[J]. Int J Coal Geol, 2017, 170(1): 77-83. -





 下载:
下载: