Effect of solvent characteristics on reaction behavior of hydroliquefaction intermediate products from Naomaohu coal
-
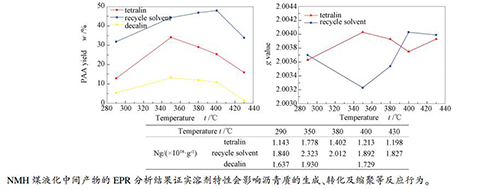
摘要: 为探究溶剂特性对煤加氢液化中间产物反应行为的影响,以新疆淖毛湖煤作为原料,四氢萘、循环溶剂及十氢萘作为供氢溶剂,在高压搅拌釜中进行直接加氢液化实验,并运用电子顺磁共振手段分析了中间产物-沥青质的自由基浓度的变化。结果表明,四氢萘溶剂中沥青质随反应温度的升高在大量生成的同时又被转化,产率从290℃的12.92%到350℃的最大34.13%再到430℃的15.98%;循环溶剂中沥青质产率先持续上升,290℃即有31.89%,400℃达到最大47.96%,之后由于结焦反应降低至33.90%。十氢萘溶剂中沥青质产率变化趋势与四氢萘一致。三种溶剂中沥青质自由基浓度的变化趋势相同,均在350℃达到最大值,分别是1.778×1018、2.323×1018和1.930×1018/g,整体上看循环溶剂数值要高于四氢萘,十氢萘介于两者之间。而四氢萘及循环溶剂中沥青质的g值在2.00323-2.00403,变化趋势与液化气体产物中COx含量变化相吻合。Abstract: To explore effect of solvent characteristics on reaction behavior of coal hydroliquefaction intermediate products, coal from Naomaohu in Xinjiang as raw material, tetralin, recycle solvent and decalin as hydrogen-donor solvents, hydroliquefaction experiments were performed in a high-pressure stirred reactor, and change of free radical concentration of asphaltene was analyzed by EPR. The results indicate that asphaltene in tetralin is formed in large quantities and transformed at the same time with increasing reaction temperature, the yield is from 12.92% at 290 ℃ to a maximum of 34.13% at 350 ℃ and then to 15.98% at 430 ℃. The asphaltene yield in recycle solvent continues to rise first, with 31.89% at 290 ℃ and a maximum of 47.96% at 400 ℃, and then decreases to 33.90% due to coking reaction. The change of asphaltene yield in decalin is consistent with that in tetralin. The change of free radical concentration of asphaltene is the same in three solvents, reaching the maximum at 350 ℃, which is 1.778×1018, 2.323×1018 and 1.930×1018/g respectively. On the whole, the values of free radical concentration of asphaltene in recycle solvent are higher than those in tetralin, and that in decalin is between the two solvents. But the g value in tetralin and recycle solvent is between 2.00323 and 2.00403, and the change is consistent with that of COx content in gas products.
-
Key words:
- Naomaohu coal /
- hydrogenation liquefaction /
- asphaltene /
- EPR /
- free radical concentration
-
表 1 NMH煤的工业分析及元素分析
Table 1 Proximate and ultimate analyses of NMH coal
Sample Rank Proximate analysisw/% Ultimate analysis wdaf/% H/C
(mol ratio)Mad Ad Vdaf FCdaf C H O* N St NMH coal lignite 15.08 5.12 52.28 47.72 73.52 5.68 19.60 0.96 0.24 0.93 *: by difference 表 2 循环溶剂组分分析
Table 2 Component analysis of recycle solvent
No. Compound Proportion/% No. Compound Proportion/% 1 pyrene 17.83 9 pyrene, 1-methyl- 3.95 2 4, 5-dihydropyrene 7.83 10 1, 2, 3, 5, 6, 7-hexahydro-4,
8-dimethyl-s-indacene2.37 3 pyrene, 1, 2, 3, 3a, 4, 5-hexahydro- 5.37 11 naphthalene, 6-butyl-1, 2, 3, 4-tetrahydro- 2.31 4 pyrene, 1, 2, 3, 6, 7, 8-hexahydro- 5.07 12 3, 3′-dimethylbiphenyl 2.22 5 anthracene, 1, 2, 3, 4, 5, 6, 7, 8-octahydro- 4.72 13 1H-Indene, 4, 7-dimethyl- 2.18 6 pyrene, hexadecahydro- 4.68 14 trans-anti-trans-perhydroanthracene 2.14 7 naphthalene, 1-(2-propenyl)- 4.64 15 nonadecane 2.10 8 heptadecane 4.37 16 others 28.22 表 3 沥青质EPR测试的实验参数
Table 3 Experimental parameters of EPR of PAA
Parameter Numerical value Parameter Numerical value Test temperature 298K scanning width 100G Microwave frequency (9.8±10 -8)GHz time constant 5.12ms Microwave power 4mW scanning time 20.97s Modulation amplitude 1G modulation frequency 100kHz Central magnetic field (3510±10 -6)G 表 4 不同溶剂中煤加氢液化沥青质的自由基浓度(Ng)
Table 4 Free radical concentration(Ng) of PAAs of coal hydroliquefaction in different solvents
Temperaturet/℃ 290 350 380 400 430 Ng/(×1018·g-1) tetralin 1.143 1.778 1.402 1.213 1.198 recycle solvent 1.840 2.323 2.012 1.892 1.827 decalin 1.637 1.930 1.729 -
[1] 郭薇.新疆淖毛湖矿区煤田地质特征及可采煤层对比研究[J].环球人文地理, 2016, (24):90. doi: 10.3969/j.issn.2095-0446.2016.24.069GUO Wei. Study on the geological characteristics and the comparison of coal seams in the coal field of Naomohu in xinjiang[J]. Geol Sur, 2016, (24):90. doi: 10.3969/j.issn.2095-0446.2016.24.069 [2] 赵正威, 李聪聪, 包志洪, 魏云迅.新疆淖毛湖矿区1号煤层煤质特征及清洁利用方向[J].中国煤炭地质, 2018, 30(9):1-4. doi: 10.3969/j.issn.1674-1803.2018.09.01ZHAO Zheng-wei, LI Cong-cong, BAO Zhi-hong, WEI Yun-xun. Coal quality features and clean utilization irientation of coal No.1 in nom nur mine area, Xinjiang[J]. Coal Geol China, 2018, 30(9):1-4. doi: 10.3969/j.issn.1674-1803.2018.09.01 [3] 高晋生, 张德祥.煤液化技术[M].北京:化学工业出版社, 2005:130-162.GAO Jin-sheng, ZHANG De-xiang. Coal Liquefaction Technology[M]. Beijing:Chemical Industry Press, 2005:130-162. [4] 周扬, 张媛媛, 陈丽诗, 潘铁英, 张德祥.两种西部煤的化学结构及加氢液化性能[J].煤炭转化, 2017, 40(6):1-6. doi: 10.3969/j.issn.1004-4248.2017.06.001ZHOU Yang, ZHANG Yuan-yuan, CHEN Li-shi, PAN Tie-ying, ZHANG De-xiang. Chemical structure and hydrogenation liquefaction performance of two kinds of western coal[J]. Coal Convers, 2017, 40(6):1-6. doi: 10.3969/j.issn.1004-4248.2017.06.001 [5] SIMSEK E H, GULEC F, KAVUSTU H. Application of Kalman filter to determination of coal liquefaction mechanisms using discrete time models[J]. Fuel, 2017, 207:814-820. doi: 10.1016/j.fuel.2017.06.004 [6] 赵鹏, 李军芳, 吴艳, 毛学锋, 张晓静, 常秋连.复杂多相体系煤加氢液化反应与氢传递的研究[J].燃料化学学报, 2018, 46(12):1423-1429. doi: 10.3969/j.issn.0253-2409.2018.12.002ZHAO Peng, LI Jun-fang, WU Yan, MAO Xue-feng, ZHANG Xiao-jing, CHANG Qiu-lian. Reaction and hydrogen transfer in complex multi-phase system during coal hydro-liquefaction[J]. J Fuel Chem Technol, 2018, 46(12):1423-1429. doi: 10.3969/j.issn.0253-2409.2018.12.002 [7] 罗化峰, 凌开成, 张卫帅, 王顺华, 冯伟, 申峻.氢气在无催化煤液化中的反应机理[J].煤炭转化, 2011, 34(4):20-24. doi: 10.3969/j.issn.1004-4248.2011.04.006LUO Hua-feng, LING Kai-cheng, ZHANG Wei-shuai, WANG Shun-hua, FENG Wei, SHEN Jun. Reaction mechanism of hydrogen for direct coal liquefaction without catalysts[J]. Coal Convers, 2011, 34(4):20-24. doi: 10.3969/j.issn.1004-4248.2011.04.006 [8] NIU B, JIN L J, LI Y, SHI Z W, HU H Q. Isotope analysis for understanding the hydrogen transfer mechanism in direct liquefaction of Bulianta coal[J]. Fuel, 2017, 203:82-89. doi: 10.1016/j.fuel.2017.04.079 [9] 李刚, 凌开成.煤高温快速液化影响因素的研究[J].燃料化学学报, 2009, 37(6):648-653. doi: 10.3969/j.issn.0253-2409.2009.06.002LI Gang, LING Kai-cheng. Influencing factors on quick coal liquefaction at high temperature[J]. J Fuel Chem Technol, 2009, 37(6):648-653. doi: 10.3969/j.issn.0253-2409.2009.06.002 [10] 宁奕飞, 张媛媛, 周扬, 陈丽诗, 潘铁英, 张德祥.反应时间对淖毛湖煤加氢液化中间产物自由基浓度影响研究[J].燃料化学学报, 2018, 46(11):1281-1287. doi: 10.3969/j.issn.0253-2409.2018.11.001NING Yi-fei, ZHANG Yuan-yuan, ZHOU Yang, CHEN Li-shi, PAN Tie-ying, ZHANG De-xiang. Effect of reaction time on free radical concentration in hydrogenation liquefaction of Naomaohu coal[J]. J Fuel Chem Technol, 2018, 46(11):1281-1287. doi: 10.3969/j.issn.0253-2409.2018.11.001 [11] 陈茺, 许学敏, 高晋生.煤中前沥青烯与沥青烯性质的研究[J].华东理工大学学报, 1998, (1):31-34. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK199800239570CHEN Chong, XU Xue-min, GAO Jin-sheng. Nature of preasphaltene and asphaltene in coal[J]. J East Chin Univ Sci Technol, 1998, (1):31-34. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK199800239570 [12] MALHOTRA V M, BUCKMASTER H A. 9 and 34 GHz EPR study of the free radicals in various asphaltenes:statistical correlation of the g-values with heteroatom content[J]. Org Geochem, 1985, 8(4):235-239. doi: 10.1016/0146-6380(85)90001-4 [13] MICHAEL G, AL-SIRI M, KHAN Z H, ALI F A. Differences in average chemical structures of asphaltene fractions separated from feed and product oils of a mild thermal processing reaction[J]. Energy Fuels, 2005, 19(4):1598-1605. doi: 10.1021/ef049854l [14] 王知彩, 陈恩生, 潘春秀, 任世彪, 雷智平, 水恒福.胜利褐煤液化沥青烯光谱表征[J].燃料化学学报, 2014, 42(6):656-661. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18425.shtmlWANG Zhi-cai, CHEN En-sheng, PAN Chun-xiu, REN Shi-biao, LEI Zhi-ping, SHUI Heng-fu. Spectral characterization of asphaltene from direct liquefaction of Shengli lignite[J]. J Fuel Chem Technol, 2014, 42(6):656-661. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18425.shtml [15] 薛永兵, 凌开成.溶剂对煤液化影响的研究[J].燃料化学学报, 2012, 40(11):1295-1299. doi: 10.3969/j.issn.0253-2409.2012.11.003XUE Yong-bing, LING Kai-cheng. Effect of solvent on direct coal liquefaction[J]. J Fuel Chem Technol, 2012, 40(11):1295-1299. doi: 10.3969/j.issn.0253-2409.2012.11.003 [16] 刘沐鑫.煤直接液化过程中溶剂的作用规律及煤裂解自由基的行为研究[D].北京: 中国科学院大学, 2015.LIU Mu-xin. The solvent action in liquefaction of coal and pyrolysis free radical behavior of coal[D]. Beijing: University of Chinese Academy of Sciences, 2015. [17] 廉鹏飞.若干潜在煤直接液化溶剂特征及对煤的辅助液化作用[D].北京: 中国科学院大学, 2017.LIAN Peng-fei. Study on characteristics of some potential coal liquefaction solvents and their effect on coal liquefaction[D]. Beijing: University of Chinese Academy of Sciences, 2017. [18] SAKATA R, TAKAYAMA A, SAKANISHI K, MOCHIDA I. Roles of nondonor solvent in the hydrogen-transferring liquefaction of Australian brown coal[J]. Energy Fuels, 1990, 4(5):585-588. doi: 10.1021/ef00023a030 [19] RUDNICK L R, TUETING D. Investigation of free radicals produced during coal liquefaction using ESR[J]. Fuel, 1984, 63(2):153-157. doi: 10.1016/0016-2361(84)90028-0 [20] DUBER S, WIECCKOWSKI A B. Effects of organic solvents on the EPR spectrum of coal[J]. Fuel, 1984, 63(12):1641-1644. doi: 10.1016/0016-2361(84)90092-9 [21] 郑榕萍. EPR定量测定煤中自由基的方法及煤液化机理的研究[D].上海: 华东理工大学, 2011.ZHENG Rong-ping. Research on quantitative determination of free radicals in coal by EPR and coal liquefaction mechanism[D]. Shanghai: East China University of Science and Technology, 2011. [22] 张德祥, 高晋生, 朱之培.年青煤在石油重油中加氢液化的研究[J].华东理工大学学报, 1986, (3):46-55. http://www.cnki.com.cn/Article/CJFDTOTAL-HLDX198603005.htmZHANG De-xiang, GAO Jin-sheng, ZHU Zhi-pei. The liquefaction of some Chinese low rank coals by hydrogenation in various heavy oils of petroleum[J]. J East Chin Univ Sci Technol, 1986, (3):46-55. http://www.cnki.com.cn/Article/CJFDTOTAL-HLDX198603005.htm [23] 刘瑞民, 夏伟平, 张德祥, 郑榕萍, 潘铁英.溶剂供氢能力对褐煤加氢及其液化产物中自由基含量的影响[C]//2010中国新型煤化工发展及示范项目进展论坛论文集.上海: 华东理工大学, 2010: 228-235.LIU Rui-min, XIA Wei-ping, ZHANG De-xiang, ZHENG Rong-ping, PAN Tie-ying. Coal liquefaction and the free racicals concentration of liquefied with the different capabitity of hydrogen-donor[C]//Papers collection of 2010 forum on development and demonstration projects of new coal chemical industry in China. Shanghai: East China University of Science and Technology, 2010: 228-235. [24] 郑榕萍, 潘铁英, 史新梅, 周丽芳, 刘瑞民, 张德祥, 高晋生.标准曲线法测定煤中自由基含量[J].波谱学杂志, 2011, 28(2):259-264. doi: 10.3969/j.issn.1000-4556.2011.02.010ZHENG Rong-ping, PAN Tie-ying, SHI Xin-mei, ZHOU Li-fang, LIU Rui-min, ZHANG De-xiang, GAO Jin-sheng. Quantitative determination of free radical content in coal by standard curve method[J]. J Mag Res, 2011, 28(2):259-264. doi: 10.3969/j.issn.1000-4556.2011.02.010 [25] 刘国根, 邱冠周.煤的ESR波谱研究[J].波谱学杂志, 1999, 16(2):177-180. doi: 10.3969/j.issn.1000-4556.1999.02.016LIU Guo-gen, QIU Guan-zhou. A study on ESR spectrum of coal[J]. J Mag Res, 1999, 16(2):177-180. doi: 10.3969/j.issn.1000-4556.1999.02.016 [26] 舒歌平, 史士东, 李克健.煤炭液化技术[M].北京:煤炭工业出版社, 2003:91-94.SHU Ge-ping, SHI Shi-dong, LI Ke-jian. Coal Liquefaction Technology[M]. Beijing:China Coal Industry Publishing House, 2003:91-94. [27] 牛犇.煤直接液化中溶剂的作用及氢传递机理[D].大连: 大连理工大学, 2017.NIU Ben. Role of solvents and hydrogen transfer mechanism in direct coal liquefaction[D]. Dalian: Dalian University of Technology, 2017. [28] NIU B, JIN L J, LI Y, SHI Z W, YAN H X, HU H Q. Interaction between hydrogen-donor and nondonor solvents in direct liquefaction of bulianta coal[J]. Energy Fuels, 2016, 30(12):10260-10267. doi: 10.1021/acs.energyfuels.6b02223 [29] 陈丽诗.煤及加氢液化中间产物结构解析与分子模型构建[D].上海: 华东理工大学, 2018.CHEN Li-shi. Structure analysis and molecular model construction of coal and its intermediate products derived from coal hydroliquefaction[D]. Shanghai: East China University of Science and Technology, 2018. [30] PETRAKIS L, GRANDY D W. Electron spin resonance spectrometric study of free radicals in coals[J]. Anal Chem, 1978, 50(2):303-308. doi: 10.1021/ac50024a034 -





 下载:
下载: