Conversion of carbon monoxide in supercritical water and the influence of alkaline potassium salts
-
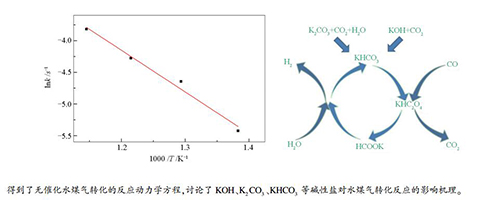
摘要: 构建了CO高压溶解的进气系统,在连续式反应系统中对超临界水条件下CO的转化规律进行了研究;针对生物质超临界水气化中钾盐的多样性,选择KHCO3、K2CO3和KOH等三种钾盐成分,研究了它们在不同工艺条件(450-600℃、23-29 MPa、停留时间3-6 s)下对超临界水中水煤气转化过程的影响。结果表明,在无催化条件下,提高反应温度、延长停留时间均提高了CO的转化率,而压力对其影响在低压下(23-25 MPa)比较大,高压下(25-29 MPa)比较小,水煤气转化的反应动力学方程为k=103.75×exp(-0.66×105/RT)(s-1)。碱性钾盐均能显著提高CO转化率,其催化促进程度从大到小依次为:KHCO3>K2CO3>KOH。添加碱性钾盐时,提高反应温度、延长停留时间均提高CO转化率,而压力的影响比较复杂。碱性盐对水煤气转化反应的催化是通过草酸盐(HC2O4-)和甲酸盐(HCOO-)作为中间产物进行的。Abstract: The conversion of carbon monoxide in supercritical water was investigated in a continuous reaction system which could dissolve carbon monoxide in water at high pressure. Meanwhile, due to the diversity of potassium salts in biomass supercritical water gasification, the influence of various alkaline potassium salts (KHCO3, K2CO3 and KOH) on the water gas shift reaction were investigated at 450-600 ℃, 23-29 MPa and with a residence time of 3-6 s. The results show that under non-catalytic conditions, the increases in reaction temperature and residence time both lead to higher CO conversion. The effect of pressure on CO conversion is distinct at low pressure (23-25 MPa), but rather minor at higher pressure (25-29 MPa). The rate expression for the non-catalytic water gas shift reaction is k=103.75×exp(-0.66×105/RT)(s-1). The potassium salts can promote the CO conversion significantly and the activity of three salts follows the order of KHCO3 > K2CO3 > KOH; the conversion of CO is enhanced at higher temperature and longer reaction time, whereas the effect of pressure on CO conversion is much complicated. The catalytic effect of alkaline potassium salts in the CO conversion may be explained by the formation of oxalate (HC2O4-) and formate (HCOO-) intermediates.
-
Key words:
- alkaline potassium salts /
- water gas shift /
- supercritical water /
- CO conversion
-
图 1 连续式超临界水反应系统示意图
Figure 1 Schematic diagram of continuous supercritical water gasification system
1: feedstock tank; 2: carbon monoxide saturator; 3: high pressure pump; 4: needle valve; 5: precooler; 6: cooling water; 7: reaction area; 8: high pressure ceramic tube; 9: electric stove wire; 10: K type thermocouple; 11: heat insulator; 12: post-cooler; 13: pressure gage; 14: 50 μ m filter; 15: 5 μ m filter; 16: back pressure valve; 17: gas collection; 18: gas-liquid separator
-
[1] RATNASAMY C, WAGNER J P. Water gas shift catalysis[J]. Catal Rev, 2009, 51(3):325-440. http://d.old.wanfangdata.com.cn/Periodical/cuihuaxb201505010 [2] MADENOĞLU T G, YILDIRIR E, SAĞLAM M, YÜKSEL M, BALLICE L. Improvement in hydrogen production from hard-shell nut residues by catalytic hydrothermal gasification[J]. J Supercrit Fluids, 2014, 95:339-347. doi: 10.1016/j.supflu.2014.09.033 [3] MELIUS C F, BERGAN N E, SHEPHERD J E. Effect of water on combustion kinetics at high pressure[C]//Proceedings of the Twenty-Third Symposium (International) on Combustion. Pittsburgh: The Combustion Institute, 1990: 217-223. https://www.sciencedirect.com/science/article/pii/S0082078406802626 [4] RICE S F, STEEPER R R, AIKEN J D. Water density effects on homogeneous water-gas shift reaction kinetics[J]. J Phys Chem A, 1998, 102(16):2673-2678. doi: 10.1021/jp972368x [5] SATO T, KUROSAWA S, SMITH JR R L, ADSCHIRI T, ARAI K. Water gas shift reaction kinetics under noncatalytic conditions in supercritical water[J]. J Supercrit Fluids, 2004, 29(1/2):113-119. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=447aea7aacf742d16a28944c2ffc8204 [6] ARAKI K, FUJIWARA H, SUGIMOTO K, OSHIMA Y, KODA S. Kinetics of water-gas shift reaction in supercritical water[J]. J Chem Eng Jpn, 2004, 37(3):443-448. doi: 10.1252/jcej.37.443 [7] 赵亮, 生物质中钾对生物质超临界水气化过程小分子中间产物转化影响研究[D].南京: 东南大学, 2013. http://www.wanfangdata.com.cn/details/detail.do?_type=degree&id=Y2627440ZHAO Liang. Study on effects of potassium existing in biomass on small molecule intermediates gasification conversion in supercritical water[D]. Nanjing: Southeast University, 2013. http://www.wanfangdata.com.cn/details/detail.do?_type=degree&id=Y2627440 [8] ELLIOTT D C, SEALOCK JR L J. Aqueous catalyst systems for the water gas shift reaction. 1. Comparative catalyst studies[J]. Ind Eng Chem Prod Res Dev, 1983, 22(3):426-431. doi: 10.1021/i300011a008 [9] ELLIOTT D C, HALLEN R T, SEALOCK JR L J. Aqueous catalyst systems for the water gas shift reaction 2. Mechanism of basic catalysis[J]. Ind Eng Chem Prod Res Dev, 1983, 22(3):431-435. doi: 10.1021/i300011a009 [10] ELLIOTT D C, SEALOCK JR L J, BUTNER R S. Aqueous catalyst systems for the water gas shift reaction 3. Continuous gas processing results[J]. Ind Eng Chem Prod Res Dev, 1986, 25(4):541-549. doi: 10.1021/i300024a007 [11] SCHUCHARDT U, SOUSA M F B. Oxalate as an intermediate in the base catalyzed water-gas shift reaction[J]. Fuel, 1986, 65(5):669-672. doi: 10.1016/0016-2361(86)90362-5 [12] AKGÜL G, KRUSE A. Influence of salts on the subcritical water-gas shift reaction[J]. J Supercrit Fluids, 2012, 64:207-214. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=299fc20af8e41bb636b6c2fcf3c095d7 [13] BARBIERB H, VINAUGER M, COLCOMBET J, EPHRITIKHINE G, FRACHISSE J M, MAUREL C. Anion channels in higher plants:functional characterization, molecular structure and physiological role[J]. Biochim Biophys Acta, 2000, 1465(1/2):199-218. https://www.sciencedirect.com/science/article/pii/S0005273600001395 [14] YASAKA Y, YOSHIDA K, WAKAI C, MATUBAYASI N, NAKAHARA M. Kinetic and equilibrium study on formic acid decomposition in relation to the water-gas-shift reaction[J]. J Phys Chem A, 2006, 110(38):11082-11090. doi: 10.1021/jp0626768 [15] 许越.化学反应动力学[M]. 1版.北京:化学工业出版社, 2005.XU Yue. Chemical Reaction Kinetics[M]. 1st ed. Beijing:Chemical Industry Press, 2005. [16] AKIYA N, SAVAGE P E. Roles of water for chemical reactions in high-temperature water[J]. Chem Rev, 2002, 102:2725-2750. doi: 10.1021/cr000668w [17] ONSAGER O T, BROWNRIGG M S A, LØDENG R. Hydrogen production from water and CO via alkali metal formate salts[J]. Int J Hydrogen Energy, 1996, 21(10):883-885. doi: 10.1016/0360-3199(96)00031-6 [18] KRUSE A, DINJUS E. Influence of salts during hydrothermal biomass gasification:The role of the catalyzed water-gas shift reaction[J]. Z Phys Chem, 2005, 219(3):341-366. -





 下载:
下载: