Structure and oxidation reactivity of char: Effects of pyrolysis heating rate and pressure
-
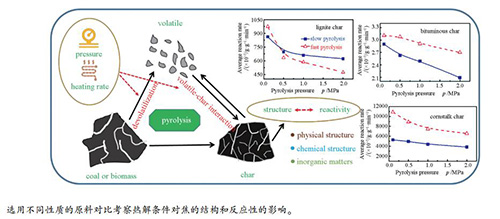
摘要: 以锡盟褐煤(L)、西山烟煤(B)和玉米秸秆(C)为原料考察了热解压力和升温速率对焦结构及氧化反应性的影响。利用两段式加压固定床反应器,在终温900℃,升温速率5℃/min和200℃/min以及压力0.1-2.0 MPa的热解条件下分别得到了慢速热解焦(SC)和快速热解焦(FC),对焦进行了比表面积、表面形貌和芳香度表征,并且采用等温热重法对焦的氧化反应性进行了分析。结果表明,热解压力和升温速率影响挥发分的停留时间和释放速率,进而影响焦的产率和性质。三种原料的热解行为不同,热解压力和升温速率对焦的产率及焦结构和反应性的影响表现出不同的特点。三种原料快速热解焦产率都低于慢速热解焦产率,且焦产率都随着压力的升高而略微上升。L-FC和B-FC的比表面积分别大于L-SC和B-SC的比表面积。C-FC的比表面积却小于C-SC的比表面积。FC的表面要比SC的表面更为粗糙。B-FC的芳香度小于B-SC的芳香度,但是在加压热解条件下L-SC和C-SC的芳香度反而分别比L-FC和C-FC的低。高压慢速热解的焦氧化反应性较差。玉米秸秆焦的氧化反应性与矿物质的催化密切相关。热解过程中升温速率和压力会影响玉米秸秆焦中矿物质的含量和分布,这也是玉米秸秆焦的氧化反应性明显高于煤焦的氧化反应性的主要原因。Abstract: Two different ranks of coals (Ximeng lignite, L and Xishan bituminous coal, B) and biomass (cornstalk, C) were selected to investigate effects of heating rate and pressure on structure and oxidation reactivity of chars from pyrolysis. The chars were prepared in a two-step pressurized fixed bed reactor at 900℃ from slow pyrolysis and fast pyrolysis under a range of pressures (0.1-2.0 MPa), which were marked as SC and FC, respectively. Specific surface area, surface morphology, and aromaticity of chars were characterized. Isothermal thermogravimetry was performed to study oxidation reactivity of chars. Results indicate that the char yields of three samples are distinctly varied and pyrolysis pressure and heating rate influence them through different residence time and diffusion rate of volatiles varying with different raw materials. The yields of chars from fast pyrolysis are all lower than those from slow pyrolysis and they increase slightly with increasing pyrolysis pressure from atmospheric pressure to 2.0 MPa. The specific surface areas of L-FC and B-FC are larger than that of L-SC and B-SC. However, the specific surface area of C-FC is smaller than that of C-SC. The morphology of FC is all rougher than that of SC. The aromaticity of B-SC is higher than that of B-FC, while that of L-SC and C-SC is lower than that of L-FC and C-FC derived from pressurized pyrolysis. The evolution of char structure at low heating rate and high pressure results generally in poor reactivity of char. The reactivity of cornstalk char is much higher than that of coal char, which should be correlated to the dispersion and concentration of inorganic matters in the char which is affected by heating rate and pressure.
-
Key words:
- heating rate /
- pyrolysis pressure /
- specific surface area /
- surface morphology /
- aromaticity /
- oxidation reactivity
-
Table 1 Proximate and ultimate analyses of raw materials
Sample Proximate analysis w/% Ultimate analysis wdaf /% Mad Ad Vdaf C H N S O* L 10.97 19.75 46.63 69.48 4.33 1.37 1.09 23.73 B 0.41 7.47 16.18 91.14 4.39 1.39 1.57 1.51 C 5.79 2.27 81.04 48.99 5.84 0.72 0.08 44.37 note: d is dry basis; ad is air dry basis; daf is dry and ash-free basis; *: by difference Table 2 Char sample IDs and preparation conditions
Sample ID Raw material Pyrolysis type Pyrolysis pressure p /MPa L-SC lignite slow pyrolysis 0.1-2.0 L-FC lignite fast pyrolysis 0.1-2.0 B-SC bituminous slow pyrolysis 0.1-2.0 B-FC bituminous fast pyrolysis 0.1-2.0 C-SC cornstalk slow pyrolysis 0.1-2.0 C-FC cornstalk fast pyrolysis 0.1-2.0 Table 3 Ash composition analysis of raw materials
Sample Composition w/% SiO2 Al2O3 Fe2O3 CaO MgO TiO2 SO3 K2O Na2O P2O5 L 57.28 12.63 5.21 7.00 2.94 0.70 9.00 1.80 1.44 0.36 B 28.57 41.21 2.70 0.54 0.22 1.23 0.50 0.52 1.43 0.03 C 40.99 1.47 2.09 3.31 2.54 0.07 3.55 30.72 0.95 4.13 -
[1] WEN Y X, XU X, XIAO Y H. Effects of pyrolysis conditions on the pore structure and reactivity of chars[J]. Proc CSEE, 2013, 33(29):63-68. [2] KAJITANI S, ZHANG Y, UMEMOTO S, ASHIZAWA M, HARA S. Co-gasification reactivity of coal and woody biomass in high-temperature gasification[J]. Energy Fuels, 2010, 24(1):2598-2609. doi: 10.1021-ef900526h/ [3] RUIZ J A, JUÁRE M C, MORALES M P, MUÑOZ P, MENDÍVIL M A. Biomass gasification for electricity generation:Review of current technology barrier[J]. Renewable Sustainable Energy Rev, 2013, 18(2):174-183. [4] MAHINPEY N, GOMEZ A. Review of gasification fundamentals and new findings:Reactors, feedstock, and kinetic studies[J]. Chem Eng Sci, 2016, 148:14-31. doi: 10.1016/j.ces.2016.03.037 [5] LI T T, ZHANG L, DONG L, QIU P H, WANG SH, JIANG SH J, LI C Z. Changes in char structure during the low-temperature pyrolysis in N2 and subsequent gasification in air of Loy Yang brown coal char[J]. Fuel, 2018, 212:187-192. doi: 10.1016/j.fuel.2017.10.026 [6] ZHANG L, LI T T, WANG SH, DONG L, LI C Z. Effects of alkali and alkaline earth metallic species and chemical structure on nascent char O2 reactivity[J]. Energy Fuels, 2017, 31(12):13578-13584. doi: 10.1021/acs.energyfuels.7b03022 [7] ZHANG Y, ZHENG Y. Co-gasification of coal and biomass in a fixed bed reactor with separate and mixed bed configurations[J]. Fuel, 2016, 183:132-138. doi: 10.1016/j.fuel.2016.06.066 [8] ROBERTS D G, HARRIS D J, WALL T F. On the effects of high pressure and heating rate during coal pyrolysis on char gasification reactivity[J]. Energy Fuels, 2003, 17(4):887-895. doi: 10.1021/ef020199w [9] TIAN B, QIAO Y Y, TIAN Y Y, LIU Q. Investigation on the effect of particle size and heating rate on pyrolysis characteristics of a bituminous coal by TG-FTIR[J]. J Anal Appl Pyrolysis, 2016, 121:376-386. doi: 10.1016/j.jaap.2016.08.020 [10] CHEN L, ZENG C, GUO X, MAO Y, ZHANG Y, ZHANG X, LI W, LONG Y, ZHU H, EITENEER B, ZAMANSKY V. Gas evolution kinetics of two coal samples during rapid pyrolysis[J]. Fuel Process Technol, 2010, 91(8):848-852. doi: 10.1016/j.fuproc.2010.02.010 [11] CETIN E, GUPTA R, MOGHTADERI B. Effect of pyrolysis pressure and heating rate on radiate pine char structure and apparent gasification reactivity[J]. Fuel, 2005, 84(10):1328-1334. doi: 10.1016/j.fuel.2004.07.016 [12] GUERRERO M, RUIZ M P, ALZUETA M U, BIBAO R, MILLERA A. Pyrolysis of eucalyptus at different heating rates:Studies of char characterization and oxidative reactivity[J]. J Anal Appl Pyrolysis, 2005, 74(1):307-314. http://cn.bing.com/academic/profile?id=67b31183b069a1029436d4bb80ab7d8c&encoded=0&v=paper_preview&mkt=zh-cn [13] LU L M, KONG CH H, SAHAJWALLA V, HARIS D. Char structural ordering during pyrolysis and combustion and its influence on char reactivity[J]. Fuel, 2002, 81(9):1215-1225. doi: 10.1016/S0016-2361(02)00035-2 [14] MERMOUD F, SALVADOR S, VAN DE STEENE L, GOLFIER F. Influence of the pyrolysis heating rate on the steam gasification rate of large wood char particles[J]. Fuel, 2006, 85(10):1473-1482. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=JJ029610249 [15] OKUMURA Y, HANAOKA Y, SAKANISHI K. Effect of pyrolysis conditions on gasification reactivity of woody biomass derived char[J]. Proc Combust Inst, 2009, 32(2):2013-2020. doi: 10.1016/j.proci.2008.06.024 [16] CETIN E, MOGHTADIRI B, GUPTA R, WALL T F. Influence of pyrolysis conditions on the structure and gasification reactivity of biomass chars[J]. Fuel, 2004, 83(16):2139-2150. doi: 10.1016/j.fuel.2004.05.008 [17] YU J L, LUCAS J A, WALL T F. Formation of the structure of chars during devolatilization of pulverized coal and its thermoproperties:A review[J]. Prog Energy Combust Sci, 2007, 33(2):135-170. doi: 10.1016/j.pecs.2006.07.003 [18] LI F H, YAN Q X, HUANG J J, ZHAO J T, FANG Y T, WANG J F. Lignite-char gasification mechanism in mixed atmospheres of steam and CO2 at different pressures[J]. Fuel Process Technol, 2015, 138:555-563. doi: 10.1016/j.fuproc.2015.06.035 [19] HARRIS D J, ROBERTS D G, HENDERSON D G. Gasification behavior of Australian coals at high temperature and pressure[J]. Fuel, 2006, 85(2):134-142. doi: 10.1016/j.fuel.2005.07.022 [20] LU L, SAHAJWALLA V, KONG C, HARRIS D. Quantitative X-ray diffraction analysis and its application to various coals[J]. Carbon, 2001, 39(12):1821-1833. doi: 10.1016/S0008-6223(00)00318-3 [21] SENNECA O, CORTESE L. Thermal annealing of coal at high temperature and high pressure. Effects on fragmentation and on rate of combustion, gasification and oxy-combustion[J]. Fuel, 2014, 116(1):221-228. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=JJ0232108835 [22] WANG M J, TIAN J L, ROBERTS D G, CHANG L P, XIE K C. Interactions between corncob and lignite during temperature programmed co-pyrolysis[J]. Fuel, 2015, 142:102-108. doi: 10.1016/j.fuel.2014.11.003 [23] WANG W L, REN X Y, CHANG J M, CAI L P, SHI S Q. Characterization of bio-oils and bio-chars obtained from the catalytic pyrolysis of alkali lignin with metal chlorides[J]. Fuel Process Technol, 2015, 138:605-611. doi: 10.1016/j.fuproc.2015.06.048 [24] LEE C W, SCARONI A W, JENKINS R D. Effect of pressure on the devolatilization and swelling behavior of a softening coal during rapid heating[J]. Fuel, 1991, 70(8):957-965. doi: 10.1016/0016-2361(91)90051-B [25] ROBERTS D G, HARRIS D J. Char gasification with O2, CO2 and H2O:Effects of pressure on intrinsic reaction kinetics[J]. Energy Fuels, 2000, 14(2):483-489. doi: 10.1021/ef9901894 [26] HOWANIEC N. The effects of pressure on coal chars porous structure development[J]. Fuel, 2016, 172:118-123. doi: 10.1016/j.fuel.2016.01.028 [27] GUERRERO M, RUIZ M P, ANGELA M, ÁNGELA M, ALZUETA M U, BILBAO R. Characterization of biomass chars formed under different devolatilization conditions:Differences between rice husk and eucalyptus[J]. Energy Fuels, 2008, 22(2):1275-1284. doi: 10.1021/ef7005589 [28] LIU Z Y, GUO X J, SHI L, HE W J, WU J F, LIU Q Y, LIU J H. Reaction of volatiles-A crucial step in pyrolysis of coals[J]. Fuel, 2015, 154:361-369. doi: 10.1016/j.fuel.2015.04.006 [29] FUSHIMI C, ARAKI K, YOHSUKE Y, TSUTSUMI A. Effect of heating rate on steam gasification of biomass. 1. Reactivity of char[J]. Ind Eng Chem Res, 2003, 42(17):3922-3928. doi: 10.1021/ie030056c [30] CETIN E, MOGHTADERI B, GUPTA R, WALL T F. Biomass gasification kinetics:influences of pressure and char structure[J]. Combust Sci Technol, 2005, 177(4):765-791 doi: 10.1080/00102200590917266 [31] LI X J, LI C Z. Volatilisation and catalytic effects of alkali and alkaline earth metallic species during the pyrolysis and gasification of Victorian brown coal. Part Ⅷ. Catalysis and changes in char structure during gasification in steam[J]. Fuel, 2006, 85(10/11):1518-1525. [32] WANG M J, ROBERTS D G, KOCHANEK M A, HARRIS D J, CHANG L P, LI C Z. Raman spectroscopic investigations into links between reactivity and char chemical structure[J]. Energy Fuels, 2014, 28(1):285-290. doi: 10.1021/ef401281h [33] TORSTEN K, CHRISTIAN L, ALEXANDER W. Qualitative evaluation of alkali release during the pyrolysis of biomass[J]. Energy Fuels, 2007, 21(5):3017-3022. doi: 10.1021/ef070094z [34] LI C Z. Importance of volatile-char interactions during the pyrolysis and gasification of low-rank fuels-A review[J]. Fuel, 2013, 112:609-623. doi: 10.1016/j.fuel.2013.01.031 [35] SATHE C, HAYASHI J L, LI C Z, CHIBA T. Release of alkali and alkaline earth metallic species during rapid pyrolysis of a victorian brown coal at elevated pressures[J]. Fuel, 2003, 82(12):1491-1497. doi: 10.1016/S0016-2361(03)00070-X -





 下载:
下载: