-
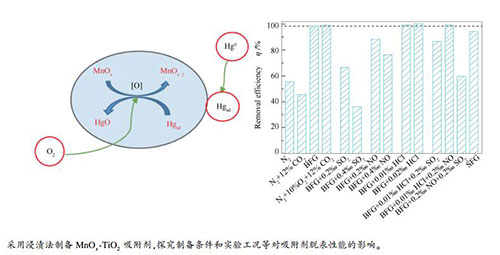
摘要: 以锐钛矿型TiO2为载体,采用浸渍法对其进行MnOx改性制备脱汞吸附剂,探究了负载量、焙烧温度、反应温度及烟气组分等参数对吸附剂脱汞性能的影响。利用N2吸附-脱附、TG/DTG、XRD、FT-IR、Hg-TPD、XPS等方法对吸附剂的理化性质进行了表征。结果表明,Mn的最佳负载量为12%,最佳焙烧温度和反应温度分别为450和300 ℃,在实验条件下MnOx-TiO2吸附剂可达到的最佳脱汞效率为98.46%。烟气中少量的O2及微量的HCl对吸附剂的脱汞有较强的促进作用;SO2对吸附剂的脱汞有较强的抑制作用,SO2与Hg0存在的竞争吸附作用以及脱汞反应中产生的硫酸盐覆盖活性位点表面,是导致脱汞效率下降的主要原因。烟气中的CO2和NO也会对汞的脱除产生轻微的抑制作用。负载在吸附剂上的MnOx存在Mn4+、Mn3+两种价态,其中,Mn4+将Hg0氧化为Hg2+,自身被还原为Mn3+。结合实验和分析结果发现,Hg0的吸附和氧化基本遵循Mars-Maessen和Langmuir-Hinshelwood机理。Abstract: A series of MnOx modified TiO2 based sorbents were synthesized using wet impregnation method and its performance on elemental mercury (Hg0) removal was studied. The effects of loading amount, calcination temperature, reaction temperature, and flue gas compositions on mercury removal efficiency were investigated. The sorbents were characterized by TGA, N2 adsorption-desorption, XRD, FT-IR, XPS and Hg-TPD. It was shown that the optimum MnOx loading value was 12%, and the optimal calcination and reaction temperature were 450 and 300 ℃, respectively. The highest mercury removal efficiency of MnOx-TiO2 sorbent was 98.46%. O2 and HCl in flue gas played a positive role in mercury removal. SO2 had a strong inhibitory effect on mercury removal, which may be due to the competition adsorption between SO2 and Hg0. At the same time, the manganese sulfate produced during the reaction covered the surface of the active site, resulting in the decrease in mercury removal efficiency. CO2 and NO in flue gas also slightly inhibited mercury removal. Mn4+ participated in oxidizing Hg0 to Hg2+, accompanied with its reduction to Mn3+. The adsorption and oxidation process of Hg0 over MnOx-TiO2 basically conformed to the Mars-Maessen mechanism and Langmuir-Hinshelwood mechanism.
-
Key words:
- simulated flue gas /
- sorbent /
- mercury removal /
- MnOx /
- TiO2
-
图 7 12Mn-Ti-450的XPS谱图
Figure 7 XPS spectra of 12Mn-Ti-450
(a): Mn 2p of fresh and spent sample; (b): O 1s of fresh and spent sample; (c): Ti 2p of fresh and spent sample; (d): Hg 4f of spent sample in BFG atmosphere; (e): Hg 4f of spent sample in (BFG+0.4‰ SO2); (f): S 2p of spent sample in (BFG+0.4‰ SO2)
表 1 Mn-Ti-450的比表面积及孔隙参数
Table 1 Surface area and pore parameters of Mn-Ti-450
Sample ABET/(m2·g-1) vt/(cm3·g-1) dave/nm TiO2 94.3 0.400 13.1 1Mn-Ti-450 90.7 0.366 11.5 3Mn-Ti-450 87.4 0.370 12.5 5Mn-Ti-450 84.3 0.373 12.4 8Mn-Ti-450 83.7 0.357 13.0 10Mn-Ti-450 81.2 0.351 13.3 12Mn-Ti-450 80.3 0.353 13.2 12Mn-Ti-400 80.2 0.366 11.5 12Mn-Ti-500 75.8 0.365 13.8 表 2 12Mn-Ti-450反应前后样品表面Mn相对含量
Table 2 Surface Mn concentration of fresh and spent 12Mn-Ti-450
Sample Surface atomic ratio /% Mn4+ Mn3+ Mn2+ Fresh 35.7 64.3 - Spent 30.5 69.5 - Spent-SO2 26.0 52.5 21.5 表 3 12Mn-Ti-450反应前后样品表面O相对含量
Table 3 Surface O concentration of fresh and spent 12Mn-Ti-450
Sample Surface atomic ratio /% Oα/(Oα+Oβ) Oβ/(Oα+Oβ) Fresh 84.4 15.6 Spent 87.9 12.1 Spent-SO2 72.4 27.6 -
[1] 郑楚光, 张军营, 赵永椿, 刘晶, 郭欣.煤燃烧汞的排放及控制[M].北京:科学出版社, 2010.ZHENG Chu-guang, ZHANG Jun-ying, ZHAO Yong-chun, LIU Jing, GUO Xin. Emission and Control of Mercury from Coal Combustion[M]. Beijing:Science Press, 2010. [2] 冯新斌, 仇广乐, 付学吾, 何天容, 李平, 王少锋.环境汞污染[J].化学进展, 2009, 21(2):436-457. http://d.old.wanfangdata.com.cn/Periodical/ysyjsjyyj201204022FENG Xin-bin, QIU Guang-le, FU Xue-wu, HE Tian-rong, LI Ping, WANG Shao-feng. Mercury pollution in the environment[J]. Prog Chem, 2009, 21(2):436-457. http://d.old.wanfangdata.com.cn/Periodical/ysyjsjyyj201204022 [3] WU Y, WANG S X, STREETS D G, HAO J M, CHAN M, JIANG J K. Trends in anthropogenic mercury emissions in China from 1995 to 2003[J]. Environ Sci Technol, 2006, 40(17):5312-5318. doi: 10.1021/es060406x [4] STREETS D G, HAO J M, WU Y, JIANG J K, CHAN M, TIAN H Z, FENG X B. Anthropogenic mercury emissions in China[J]. Atmos Environ, 2005, 39(40):7789-7806. doi: 10.1016/j.atmosenv.2005.08.029 [5] HU D, ZHANG W, CHEN L, CHEN C, OU L B, TONG Y D, WEI W, LONG W J, WANG X J. Mercury emissions from waste combustion in China from 2004 to 2010[J]. Atmos Environ, 2012, 62:359-366. doi: 10.1016/j.atmosenv.2012.08.061 [6] SENIOR C L, HRLBLE J J, SAROFIM A F. Emissions of mercury, trace elements, and fine particles from stationary combustion sources[J]. Fuel Process Technol, 2000, 65/66:263-288. doi: 10.1016/S0378-3820(00)00082-5 [7] GALBREATH K C, ZYGARLICKE C J. Mercury transformations in coal combustion flue gas[J]. Fuel Process Technol, 2000, 65-66:289-310. doi: 10.1016/S0378-3820(99)00102-2 [8] 王运军, 段钰锋, 杨立国, 孟素丽, 黄治军, 吴成军, 王乾.湿法烟气脱硫装置和静电除尘器联合脱除烟气中汞的试验研究[J].中国电机工程学报, 2008, 28(29):64-69. doi: 10.3321/j.issn:0258-8013.2008.29.012WANG Yun-jun, DUAN Yu-feng, YANG Li-guo, MENG Su-li, HUANG Zhi-jun, WU Cheng-jun, WANG Qian. Experimental study on mercury removal by combined wet flue gas desulphurization with electrostatic precipitator[J]. Proc CSEE, 2008, 28(29):64-69. doi: 10.3321/j.issn:0258-8013.2008.29.012 [9] 胡长兴, 周劲松, 骆仲泱, 高洪亮, 王勤辉, 岑可法.烟气脱汞过程中活性炭喷射量的影响因素[J].化工学报, 2005, 56(11):140-145. http://d.old.wanfangdata.com.cn/Periodical/hgxb200511026HU Chang-xing, ZHOU Jin-song, LUO Zhong-yang, GAO Hong-liang, WANG Qin-hui, CEN Ke-fa. Factors affecting amount of activated carbon injection for flue gas mercury control[J]. J Chem Ind Eng (China), 2005, 56(11):140-145. http://d.old.wanfangdata.com.cn/Periodical/hgxb200511026 [10] SJOSTROM S, DURHAM M, BUSTARD C J, MARTIN C. Activated carbon injection for mercury control:Overview[J]. Fuel, 2010, 89(6):1320-1322. doi: 10.1016/j.fuel.2009.11.016 [11] DING F, ZHAO Y C, MI L L, LI H L, LI Y, ZHANG J Y. Removal of gas-phase elemental mercury in flue gas by inorganic chemically promoted natural mineral sorbents[J]. Ind Eng Chem Res, 2012, 51(7):3039-3047. doi: 10.1021/ie202231r [12] 任建莉, 周劲松, 骆仲泱, 胡长兴, 钟英杰.新型吸附剂脱除烟气中气态汞的试验研究[J].中国电机工程学报, 2007, 27(2):48-53. doi: 10.3321/j.issn:0258-8013.2007.02.010REN Jian-li, ZHOU Jin-song, LUO Zhong-yang, HU Chang-xing, ZHONG Ying-jie. The application of novel sorbents for mercury vapor removal from simulated flue gases[J]. Proc CSEE, 2007, 27(2):48-53. doi: 10.3321/j.issn:0258-8013.2007.02.010 [13] SHAO H Z, LIU X W, ZHOU Z J, ZHAO B, CHEN Z G, XU M H. Elemental mercury removal using a novel KI modified bentonite supported by starch sorbent[J]. Chem Eng J, 2016, 291:306-316. doi: 10.1016/j.cej.2016.01.090 [14] 施冬雷, 乔仁静, 许琦.酸改性凹凸棒土的制备及其脱汞性能[J].合成化学, 2015, 23(8):720-724. http://d.old.wanfangdata.com.cn/Periodical/hchx201508010SHI Dong-lei, QIAO Ren-jing, XU Qi. Preparation of acid modified attapulgite and its performance of mercury removal[J]. Chin J Syn Chem, 2015, 23(8):720-724. http://d.old.wanfangdata.com.cn/Periodical/hchx201508010 [15] 张安超, 向军, 孙路石, 胡松, 付鹏, 程伟, 邱建荣.新型改性吸附剂制备、表征及脱除单质汞的实验研究[J].化工学报, 2009, 60(6):1546-1553. doi: 10.3321/j.issn:0438-1157.2009.06.032ZHANG An-chao, XIANG Jun, SUN Lu-shi, HU Song, FU Peng, CHENG Wei, QIU Jian-rong. Synthesis and characterization of novel modified sorbents and their performance for elemental mercury removal[J]. CIESC J, 2009, 60(6):1546-1553. doi: 10.3321/j.issn:0438-1157.2009.06.032 [16] HE J, REDDY G K, THIEL S W, SMIRNIOTIS P G, PINTO N G. Ceria-modified manganese oxide/titania materials for removal of elemental and oxidized mercury from flue gas[J]. J Phys Chem C, 2011, 115(49):24300-24309. doi: 10.1021/jp208768p [17] ZHANG X P, CUI Y Z, TAN B J, WANG J X, LI Z F, HE G H. The adsorption and catalytic oxidation of the element mercury over cobalt modified Ce-ZrO2 catalyst[J]. RSC Adv, 2016, 6(91):88332-88339. doi: 10.1039/C6RA19450H [18] DU W, YIN L B, ZHUO Y Q, XU Q S, ZHANG L, CHEN C H. Performance of CuOx-neutral Al2O3 sorbents on mercury removal from simulated coal combustion flue gas[J]. Fuel Process Technol, 2015, 131:403-408. doi: 10.1016/j.fuproc.2014.11.039 [19] WU Z B, JIANG B Q, LIU Y. Effect of transition metals addition on the catalyst of manganese/titania for low-temperature selective catalytic reduction of nitric oxide with ammonia[J]. App Catal B:Environ, 2008, 79(4):347-355. doi: 10.1016/j.apcatb.2007.09.039 [20] 谢江坤.锰基复合氧化物及其对零价汞的吸附性能研究[D].上海: 上海交通大学, 2013. http://www.wanfangdata.com.cn/details/detail.do?_type=degree&id=D575614XIE Jiang-kun. Study of manganese-based binary-metal oxides and their capability for gaseous elemental mercury capture[D].Shanghai: Shanghai Jiao Tong University, 2013. http://www.wanfangdata.com.cn/details/detail.do?_type=degree&id=D575614 [21] ZHANG S B, ZHAO Y C, WANG Z H, ZHANG J Y, WANG L L, ZHENG C G. Integrated removal of NO and mercury from coal combustion flue gas using manganese oxides supported on TiO2[J]. J Environ Sci, 2017, 53:141-150. doi: 10.1016/j.jes.2015.10.038 [22] LI H L, WU C Y, LI Y, ZHANG J Y. Superior activity of MnOx-CeO2/TiO2 catalyst for catalytic oxidation of elemental mercury at low flue gas temperatures[J]. App Catal B:Environ, 2012, 111/112:381-388. doi: 10.1016/j.apcatb.2011.10.021 [23] 刘颖, 葛培文, 苏少奎, 张丽娟, 王云平. Mn12-Ac磁性分子团簇单晶的热重-差热分析与结构转变[J].物理学报, 2004, 53(11):4015-4020. http://d.old.wanfangdata.com.cn/Periodical/wlxb200411072LIU Ying, GE Pei-wen, SU Shao-kui, ZHANG Li-juan, WANG Yun-ping. Thermal analysis and structure transformation of Mn12-Ac magnetic molecular crystals[J]. Acta Phys Sin, 2004, 53(11):4015-4020. http://d.old.wanfangdata.com.cn/Periodical/wlxb200411072 [24] 廖伟平, 杨柳, 王飞, 胡宇峰, 盛重义.不同制备方法的Mn-Ce催化剂低温SCR性能研究[J].化学学报, 2011, 69(22):2723-2728. http://d.old.wanfangdata.com.cn/Periodical/hxxb201122014LIAO Wei-ping, YANG Liu, WANG Fei, HU Yu-feng, SHENG Zhong-yi. Performance study for low-temperature SCR catalysts based on Mn-Ce prepared by different methods[J]. Acta Chim Sin, 2011, 69(22):2723-2728. http://d.old.wanfangdata.com.cn/Periodical/hxxb201122014 [25] 刘纳, 何峰, 谢峻林, 胡华, 李凤祥, 方德. Fe掺杂Mn/TiO2低温脱硝催化剂的催化性能研究[J].人工晶体学报, 2017, 46(3):490-494. doi: 10.3969/j.issn.1000-985X.2017.03.019LIU Na, HE Feng, XIE Jun-lin, HU Hua, LI Feng-xiang, FANG De. Catalytic performance of Fe-doped Mn/TiO2 catalysts for low-temperature denitration[J]. J Syn Crys, 2017, 46(3):490-494. doi: 10.3969/j.issn.1000-985X.2017.03.019 [26] XIE J K, YAN N Q, YANG S J, QU Z, CHEN W M, ZHANG W Q, LI K H, LIU P, JIA J P. Synthesis and characterization of nano-sized Mn-TiO2 catalysts and their application to removal of gaseous elemental mercury[J]. Res Chem Intermed, 2012, 38(9):2511-2522. doi: 10.1007/s11164-012-0568-z [27] JENSEN H, SOLOVIEV A, LI Z S, SΦGAARD E G. XPS and FTIR investigation of the surface properties of different prepared titania nano-powders[J]. Appl Sur Sci, 2005, 246(1):239-249. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=ca5238caa1e1c9ff8b537f3785322998 [28] ZHANG A C, XING W B, ZHANG Z H, MENG F M, LIU Z C, XIANG J, SUN L S. Promotional effect of SO2 on CeO2-TiO2 material for elemental mercury removal at low temperature[J]. Atmos Pollut Res, 2016, 7(5):895-902. doi: 10.1016/j.apr.2016.05.003 [29] OSEGHE E O, NDUNGU P G, JONNALAGADDA S B. Synthesis of mesoporous Mn/TiO2 nanocomposites and investigating the photocatalytic properties in aqueous systems[J]. Environ Sci Pollut Res Inter, 2014, 22(1):211. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=915202cba101e107f1ff6b533f4f3142 [30] 朱少文, 沈伯雄, 池桂龙, 张笑.铁钴共掺杂的Mn-Ce/TiO2催化剂低温SCR脱硝[J].环境工程学报, 2017, 11(6):3633-3639. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hjwrzljsysb201706043ZHU Shao-wen, SHEN Bo-xiong, CHI Gui-long, ZHANG Xiao. Low-temperature SCR of NO over Fe and Co co-doped Mn-Ce/TiO2 catalyst[J]. Chin J Environ Eng, 2017, 11(6):3633-3639. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hjwrzljsysb201706043 [31] ZHANG X, SHEN B X, SHEN F, ZHANG X Q, SI M, YUAN P. The behavior of the manganese-cerium loaded metal-organic framework in elemental mercury and NO removal from flue gas[J]. Chem Eng J, 2017, 326:551-560. doi: 10.1016/j.cej.2017.05.128 [32] ZHOU Q, LEI Y, LIU Y B, TAO X, LU P, DUAN Y F, WANG Y J. Gaseous elemental mercury removal by magnetic Fe-Mn-Ce sorbent in simulated flue gas[J]. Energy Fuels, 2018, 32(12):12780-12786. doi: 10.1021/acs.energyfuels.8b03445 [33] KANG M, PARK E D, KIM J M, YIE J E. Manganese oxide catalysts for NOx reduction with NH3 at low temperatures[J]. Appl Catal A:Gen, 2007, 327(2):261-269. doi: 10.1016/j.apcata.2007.05.024 [34] 李海龙.新型SCR催化剂对汞的催化氧化机制研究[D].武汉: 华中科技大学, 2011. http://www.wanfangdata.com.cn/details/detail.do?_type=degree&id=D201456LI Hai-long. Catalytic oxidation of elemental mercury over novel SCR catalysts[D]. Wuhan: Huazhong University of Science and Technology, 2011. http://www.wanfangdata.com.cn/details/detail.do?_type=degree&id=D201456 [35] YANG S J, QI F H, XIONG S C, DANG H, LIAO Y, WONG P K, LI J H. MnOx supported on Fe-Ti spinel:A novel Mn based low temperature SCR catalyst with a high N2 selectivity[J]. Appl Catal B:Environ, 2016, 181:570-580. doi: 10.1016/j.apcatb.2015.08.023 [36] YANG S J, GUO Y F, YAN N Q, QU Z, XIE J K, YANG C, JIA J P. Capture of gaseous elemental mercury from flue gas using a magnetic and sulfur poisoning resistant sorbent Mn/γ-Fe2O3 at lower temperatures[J]. J Hazard Mater, 2011, 186(1):508-515. doi: 10.1016/j.jhazmat.2010.11.034 [37] LI H H, WANG Y, WANG S K, WANG X, HU J J. Removal of elemental mercury in flue gas at lower temperatures over Mn-Ce based materials prepared by co-precipitation[J]. Fuel, 2017, 208:576-586. doi: 10.1016/j.fuel.2017.07.061 [38] 周琪琪, 王学谦, 宁平, 黄红旗, 陶雷. FeCl3改性MOFs在低温下对Hg0的吸附性能[J].环境科学研究, 2018, 31(3):528-536. http://d.old.wanfangdata.com.cn/Periodical/hjkxyj201803015ZHOU Qi-qi, WANG Xue-qian, NING Ping, HUANG Hong-qi, TAO Lei. Adsorption performance of elemental mercury on MOFs modified with FeCl3 at low temperatures[J]. Res Environ Sci, 2018, 31(3):528-536. http://d.old.wanfangdata.com.cn/Periodical/hjkxyj201803015 [39] 王红妍, 王宝冬, 李俊华, 孙琦.燃煤烟气中单质汞的催化氧化技术研究进展[J].材料导报, 2017, 31(7):114-120. http://d.old.wanfangdata.com.cn/Periodical/cldb201707018WANG Hong-yan, WANG Bao-dong, LI Jun-hua, SUN Qi. Progress in catalytic oxidation technology for element mercury in coal-fired flue gas[J]. Mater Rev, 2017, 31(7):114-120. http://d.old.wanfangdata.com.cn/Periodical/cldb201707018 [40] LIU D J, ZHOU W G, WU J. Effect of Ce and La on the activity of CuO/ZSM-5 and MnOx/ZSM-5 composites for elemental mercury removal at low temperature[J]. Fuel, 2017, 194:115-122. doi: 10.1016/j.fuel.2016.12.076 [41] DESHETTI J, SAMUEL J I, YLIAS M S, BENJARAM M R, SURESH K B. Highly efficient nanosized Mn and Fe codoped ceria-based solid solutions for elemental mercury removal at low flue gas temperatures[J]. Catal Sci Technol, 2015, 5(5):2913-2924. doi: 10.1039/C5CY00231A [42] QIAO S H, CHEN J, LI J F, QU Z, LIU P, YAN N Q, JIA J P. Adsorption and catalytic oxidation of gaseous elemental mercury in flue gas over MnOx/Alumina[J]. Ind Eng Chem Res, 2009, 48(7):3317-3322. doi: 10.1021/ie801478w [43] ZHANG S B, ZHAO Y C, YANG J P, ZHANG Y, SUN P, YU X H, ZHANG J Y, ZHENG C G. Simultaneous NO and mercury removal over MnOx/TiO2 catalyst in different atmospheres[J]. Fuel Process Technol, 2017, 166:282-290. doi: 10.1016/j.fuproc.2017.06.011 [44] TAO S S, LI C T, FAN X P, ZENG G M, LU P, ZHANG X, WEN Q B, ZHAO W W, LUO D Q, FAN C Z. Activated coke impregnated with cerium chloride used for elemental mercury removal from simulated flue gas[J]. Chem Eng J, 2012, 210:547-556. doi: 10.1016/j.cej.2012.09.028 [45] CHEN W M, PEI Y, HUANG W J, QU Z, HU X F, YAN N Q. Novel effective catalyst for elemental mercury removal from coal-fired flue gas and the mechanism investigation[J]. Environ Sci Technol, 2016, 50(5):2564-2572. doi: 10.1021/acs.est.5b05564 [46] 朱磊, 李海龙, 赵永椿, 张军营.纳米硫化锌吸附脱除单质汞的实验研究[J].工程热物理学报, 2017, 38(1):203-207. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=gcrwlxb201701036ZHU Lei, LI Hai-long, ZHAO Yong-chun, ZHANG Jun-ying. Experimental study on Hg0 adsorption by nano-ZnS[J]. J Eng Thermo, 2017, 38(1):203-207. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=gcrwlxb201701036 [47] 袁媛, 张军营, 赵永椿, 王宇翔, 郑楚光. SO2和NO浓度对TiO2-硅酸铝纤维脱除元素汞的影响[J].燃料化学学报, 2012, 40(5):630-635. doi: 10.3969/j.issn.0253-2409.2012.05.020YUAN Yuan, ZHANG Jun-ying, ZHAO Yong-chun, WANG Yu-xiang, ZHENG Chu-guang. Effects of SO2 and NO on removal of elemental mercury using a TiO2-aluminum silicate fiber[J]. J Fuel Chem Technol, 2012, 40(5):630-635. doi: 10.3969/j.issn.0253-2409.2012.05.020 [48] 孔凡海.铁基纳米吸附剂烟气脱汞实验及机理研究[D].武汉: 华中科技大学, 2010. http://www.wanfangdata.com.cn/details/detail.do?_type=degree&id=D185934KONG Fan-hai. Study on experimental and mechanism of mercury removal from flue gas with Fe based nano-sorbents[D].Wuhan: Huazhong University of Science and Technology, 2010. http://www.wanfangdata.com.cn/details/detail.do?_type=degree&id=D185934 [49] 王钧伟, 张庆平, 沈园园, 董彦杰, 张先龙, 秦伟, 张元广.凹凸棒石负载CuO催化剂脱除气态Hg0[J].环境化学, 2017, 36(5):1097-1103. http://d.old.wanfangdata.com.cn/Periodical/hjhx201705019WANG Jun-wei, ZHANG Qing-ping, SHEN Yuan-yuan, DONG Yan-jie, ZHANG Xian-long, QIN Wei, ZHANG Yuan-guang. Removal of vapor-phase Hg0 over a CuO/PG catalyst[J]. Environ Chem, 2017, 36(5):1097-1103. http://d.old.wanfangdata.com.cn/Periodical/hjhx201705019 [50] ZHAO L K, LI C T, ZHANG X N, ZENG G M, ZHANG J, XIE Y E. A review on oxidation of elemental mercury from coal-fired flue gas with selective catalytic reduction catalysts[J]. Catal Sci Technol, 2015, 5(7):3459-3472. doi: 10.1039/C5CY00219B [51] LI H L, WU C Y, LI Y, LI L Q, ZHAO Y C, ZHANG J Y. Role of flue gas components in mercury oxidation over TiO2 supported MnOx-CeO2 mixed-oxide at low temperature[J]. J Hazard Mater, 2012, 243:117-123. doi: 10.1016/j.jhazmat.2012.10.007 [52] WU Y H, XU W Q, YANG Y, WANG J, ZHU T Y. Support effect of Mn-based catalysts for gaseous elemental mercury oxidation and adsorption[J]. Catal Sci Technol, 2018, 8(1):236-297. doi: 10.1039/C7CY01351E [53] LOPEZ-ANTON M A, YUAN Y, PERRY R, MAROTO-VALER M M. Analysis of mercury species present during coal combustion by thermal desorption[J]. Fuel, 2010, 89(3):629-634. doi: 10.1016-j.fuel.2009.08.034/ [54] 杨应举, 张保华, 刘晶, 王震, 苗森.可再生循环利用CuxMn(3-x)O4尖晶石吸附剂脱汞性能[J].燃烧科学与技术, 2017, 23(6):511-515. http://d.old.wanfangdata.com.cn/Periodical/rskxyjs201706006YANG Ying-ju, ZHANG Bao-hua, LIU Jing, WANG Zhen, MIAO Sen. Mercury removal by recyclable and regenerable CuxMn(3-x)O4 spinel-type sorbents[J]. J Combus Sci Technol, 2017, 23(6):511-515. http://d.old.wanfangdata.com.cn/Periodical/rskxyjs201706006 [55] EVAN J, GRANITE, PENNLINE H W, HARGIS R A. Novel sorbents for mercury removal from flue gas[J]. Ind Eng Chem Res, 2000, 39:1020-1029. doi: 10.1021/ie990758v -





 下载:
下载: