Preparation of ZrO2 modified Al2O3 nano-sheets supported cobalt catalyst and its performance in Fischer-Tropsch synthesis
-
摘要: 通过水热法合成了Al2O3纳米片(Al2O3-CN),采用浸渍法制备20%(质量分数)钴基催化剂,并应用于费托合成反应。制备的Al2O3-CN(226 m2/g)与商业氧化铝(Al2O3-C,249 m2/g)具有相近的比表面积,但Al2O3-CN孔尺寸分布更加集中。浸渍钴后,与Co/Al2O3-C催化剂相比,Co/Al2O3-CN催化剂表现出较高的还原度及更均匀的钴颗粒粒径分布。因此,Co/Al2O3-CN催化剂表现出更高的CO转化率和低的甲烷选择性。为了进一步提高Co/Al2O3-CN的催化性能,采用不同含量ZrO2对Al2O3-CN进行修饰。表征结果表明,随着ZrO2修饰量的增加,Al2O3-CN载体比表面积变化不明显,孔体积和孔径增大;相对应催化剂的钴颗粒粒径减小,活性位点数目增加。在相同反应条件下,经ZrO2修饰催化剂CO转化率进一步提高,甲烷选择性降低。Abstract: Al2O3 nano-sheet (Al2O3-CN) was synthesized under hydrothermal condition. The cobalt-based catalyst of 20% (mass fraction) was prepared by impregnation method and applied to Fischer-Tropsch synthesis. The Al2O3-CN (226 m2/g) and commercial alumina (Al2O3-C, 249 m2/g) have similar specific surface area, but Al2O3-CN has more narrow pore size distribution. Compared with Co/Al2O3-C catalyst, Co/Al2O3-CN catalyst showed higher reduction degree and more uniform cobalt particle size distribution after impregnation. Thus, Co/Al2O3-CN catalyst exhibited higher CO conversion and lower methane selectivity. In order to further improve the catalytic performance of Co/Al2O3-CN, Al2O3-CN was modified with ZrO2. The characterization results showed that with the increase of ZrO2, the specific surface of Al2O3-CN did not change significantly, and the pore volume and pore diameter increased. The cobalt particle size decreased and the number of active sites increased. Under the same reaction conditions, the CO conversion rate of catalysts modifield by ZrO2 was farther improved and selectivity of methane was decreased.
-
Key words:
- Fischer-Tropsch synthesis /
- zirconium additives /
- alumina /
- nano-sheets
-
表 1 ZrO2在载体中的含量分析
Table 1 Quantitative analysis of the ZrO2 in the supports
Catalyst ZrO2 w/% XPS ICP Al2O3-2.5Zr-CN 11.9 2.2 Al2O3-5Zr-CN 14.6 5.7 Al2O3-7.5Zr-CN 15.3 7.5 Al2O3-10Zr-CN 17.4 10.1 表 2 载体和催化剂的物化性质
Table 2 Physico-chemical properties of the supports and the catalysts
Sample ABET/
(m2·g-1)vpore/
(cm3·g-1)dpore/
nmCo crystalline a
/nmH2-TPR reducibilityb/% H2-TPD dispersionc
/%Co0accd
(10-2mol·gcat-1)Al2O3-C 249 0.75 10.1 - - - - Al2O3-CN 226 0.29 6.3 - - - - Al2O3-2.5Zr-CN 186 0.29 7.5 - - - - Al2O3-5Zr-CN 179 0.29 6.3 - - - - Al2O3-7.5Zr-CN 181 0.26 7.1 - - - - Al2O3-10Zr-CN 192 0.28 6.8 - - - - Co/Al2O3-C 176 0.49 10.3 9.3 30.1 7.5 5.06 Co/Al2O3-CN 142 0.25 10.6 10.6 36.0 5.5 3.70 Co/Al2O3-2.5Zr-CN 138 0.35 11.7 9.9 38.9 6.0 4.04 Co/Al2O3-5Zr-CN 137 0.35 12.8 9.4 40.3 6.1 4.16 Co/Al2O3-7.5Zr-CN 146 0.39 15.0 8.9 42.8 6.6 4.50 Co/Al2O3-10Zr-CN 132 0.31 13.3 8.6 38.9 6.7 4.60 a: d(Co) = 0.75 d(Co3O4); b: calculated by H2-TPR from 373 to 673 K; c, d: calculated from H2 chemisorption 表 3 催化剂的表面组成
Table 3 Surface composition of the catalysts
Catalyst Co 2p3/2 EB/eV ICSS/
ICo3O4Co3O4 cobalt surface species Co/Al2O3-C 780.085 781.898 0.862 Co/Al2O3-CN 780.228 782.070 0.786 Co/Al2O3-2.5Zr-CN 780.202 782.034 0.736 Co/Al2O3-5Zr-CN 780.160 782.031 0.716 Co/Al2O3-7.5Zr-CN 780.109 781.828 0.697 Co/Al2O3-10Zr-CN 780.039 781.927 0.712 表 4 不同催化剂的费托反应性能
Table 4 Performance of different catalysts on Fischer-Tropsch synthesis
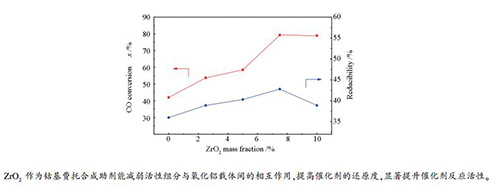
Catalyst Temperature t/℃ CO conversion x/% Hydrocarbon selectivity s/% TOF/(10-3·s-1) CH4 C2-4 C5+ Co/Al2O3-C 200 22.8 13.9 12.0 74.1 5.0 210 49.6 14.1 13.1 72.8 10.7 Co/Al2O3-CN 200 42.0 8.0 10.0 81.9 13.2 Co/Al2O3-2.5Zr-CN 200 53.7 7.3 9.2 83.5 16.4 Co/Al2O3-5Zr-CN 200 58.5 6.8 9.0 84.2 18.2 Co/Al2O3-7.5Zr-CN 200 79.3 6.5 8.9 84.6 20.7 Co/Al2O3-10Zr-CN 200 78.9 7.6 8.7 83.7 20.3 reaction conditions: H2/CO(molar ratio)=2.0, GHSV=1000 h-1, p=2.0 MPa, time on stream=48 h -
[1] MUNNIK P, DE JONGH P E, DE JONG K P. Recent developments in the synthesis of supported catalysts[J]. Chem Rev, 2015, 115(4):6687-6718. http://d.old.wanfangdata.com.cn/OAPaper/oai_doaj-articles_3e70e18b52824f132575789a5b05aec6 [2] 孙予罕, 陈建刚, 王俊刚, 贾丽涛, 侯博, 李德宝, 张娟.费托合成钴基催化剂的研究进展[J].催化学报, 2010, 31(8):919-927. http://d.old.wanfangdata.com.cn/Periodical/cuihuaxb201008007SUN Yu-han, CHEN Jian-gang, WANG Jun-gang, JIA Li-tao, HOU Bo, LI De-bao, ZHANG Juan. The development of cobalt-based catalysts for fischer-tropsch synthesis[J]. Chin J Catal, 2010, 31(8):919-927. http://d.old.wanfangdata.com.cn/Periodical/cuihuaxb201008007 [3] KHODAKOV A Y, CHU W, FONGARLAND P. Advances in the development of novel cobalt fischer-tropsch catalysts for synthesis of long-chain hydrocarbons and clean fuels[J]. Chem Rev, 2007, 107(5):1692-1744. doi: 10.1021/cr050972v [4] STORSÆTER S, TØTDAL B, WALMSLEY J C, TANEM B S, HOLMEN A. Characterization of alumina-, silica-, and titania-supported cobalt Fischer-Tropsch catalysts[J]. J Catal, 2005, 236(1):139-152. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=a4d1ef16486bb9da1e2aa82e2c22f67a [5] 李金林, 完友军, 张煜华, 熊海峰.不同载体负载的钴基费托合成催化剂的还原过程研究[J].中南民族大学学报, 2007, 26(2):1-6. doi: 10.3969/j.issn.1672-4321.2007.02.001LI Jin-lin, WAN You-jun, ZHANG Yu-hua, XIONG Hai-feng. Studies on the reduction process of the supported cobalt catalysis for Fischer-Tropsch Synthesis[J]. J South-Cent Univ Nat(Nat Sci Ed), 2007, 26(2):1-6. doi: 10.3969/j.issn.1672-4321.2007.02.001 [6] 李家波, 林泉.费托合成钴催化剂载体改性研究进展[J].洁净煤技术, 2015, 21(1):65-68. http://d.old.wanfangdata.com.cn/Periodical/jjmjs201501016LI Jia-bo, LIN Quan. Supporter modification of Fischer-Tropsch cobalt catalyst[J]. Clean Coal Technol, 2015, 21(1):65-68. http://d.old.wanfangdata.com.cn/Periodical/jjmjs201501016 [7] BAO A, LIEW K Y, LI J L. Fischer-Tropsch synthesis on CaO-promoted Co/Al2O3 catalysts[J]. J Mol Catal A:Chem, 2009, 304(1/2):47-51. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=294745cef25aebc9a5d9500787f61030 [8] YUAN Q, YIN A X, LUO C, SUN L D, ZHANG Y W, DUAN W T, LIU H C, YAN C H. Facile synthesis for ordered mesoporous γ-aluminas with high thermal stability[J]. J Am Chem Soc, 2008, 130(11):3465-3472. doi: 10.1021/ja0764308 [9] LI X, HAN D Z, XU Y Q, LIU X M, YAN Z F. Bimodal mesoporous γ-Al2O3:A promising support for CoMo-based catalyst in hydrodesulfurization of 4, 6-DMDBT[J]. Mater Lett, 2011, 65(12):1765-1767. doi: 10.1016/j.matlet.2011.03.037 [10] MARTÍNEZ A, PRIETO G, ROLLAN J. Nanofibrous γ-Al2O3 as support for cobased Fischer-Tropsch catalysts:Pondering the relevance of diffusional and dispersion effects on catalytic performance[J]. J Catal, 2009, 263(2):292-305. [11] LIU C C, LI J L, ZHANG Y H, CHEN S F, ZHU J J, LIEW K Y. Fischer-Tropsch synthesis over cobalt catalysts supported on nanostructured alumina with various morphologies[J]. J Mol Catal A:Chem, 2012, 363/364:335-342. doi: 10.1016/j.molcata.2012.07.009 [12] WANG W W, ZHOU J B, ZHANG Z, YU J G, CAI W Q. Different surfactants-assisted hydrothermal synthesis of hierarchical γ-Al2O3 and its adsorption performances for parachlorophenol[J]. Chem Eng J, 2013, 233:168-175. doi: 10.1016/j.cej.2013.08.029 [13] NABAHO D, NIEMANTSVERDRIET J W, CLAEYS M, STEEN E. Hydrogen spillover in the Fischer-Tropsch synthesis:An analysis of platinum as a promoter for cobalt-alumina catalysts[J]. Catal Today, 2016, 261:17-27. doi: 10.1016/j.cattod.2015.08.050 [14] JACOBS G, CHANRY J A, PATTERSON P M, DAS T K, DAVIS B H. Fischer-Tropsch synthesis:Study of the promotion of Re on the reduction property of Co/Al2O3 catalysts by in situ EXAFS/XANES of Co K and Re LⅢ edges and XPS[J]. Appl Catal A:Gen, 2004, 264(2):203-212. doi: 10.1016/j.apcata.2003.12.049 [15] JONGSOMJIT B, PANPRANOT J, GOODWIN JR J G. Effect of zirconia-modified alumina on the properties of Co/γ-Al2O3 catalysts[J]. J Catal, 2003, 215(1):66-77. http://www.sciencedirect.com/science/article/pii/S0021951702001021 [16] ZHANG Y H, XIONG H F, LIEW K Y, LI J L. Effect of magnesia on alumina-supported cobalt Fischer-Tropsch synthesis catalysts[J]. J Mol Catal A:Chem, 2005, 237(1/2):172-181. http://www.sciencedirect.com/science/article/pii/S1381116905002980 [17] DAI X P, YU C C, SHEN S K. Promotion effect of ceria on Fischer-Tropsch synthesis performance over Co/Al2O3 catalyst[J]. Chin J Catal, 2001, 22:104-108. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=cuihuaxb200102003 [18] MA C L, CHANG Y L, YE W C, DUAN L Y, WANG C M. Hexagon γ-alumina nanosheets produced with the assistance of supercritical ethanol drying[J]. J Supercrit Fluids, 2008, 45:112-120. doi: 10.1016/j.supflu.2008.01.001 [19] LI G C, GUAN L L, LIU Y Q, LIU C G. Template-free solvothermal synthesis of 3D hierarchical nanostructured boehmite assembled by nanosheets[J]. J Phys Chem Solids, 2012, 73:1055-1060. doi: 10.1016/j.jpcs.2012.04.014 [20] YANG Y F, JIA L T, MENG Y, HOU B, LI D B, SUN Y H. Fischer-Tropsch synthesis over ordered mesoporous carbon supported cobalt catalysts:The role of amount of carbon precursor in catalytic performance[J]. Catal Lett, 2012, 142(2):195-204. doi: 10.1007/s10562-011-0747-3 [21] KOGELBAUER A, GOODWIN J G, QUKACI R. Ruthenium promotion of Co/Al2O3 Fischer-Tropsch catalysts[J]. J Catal, 1996, 160(1):125-133. https://www.sciencedirect.com/science/article/pii/S002195179690130X [22] SEXTON B A, HUGHES A E, TURNEY T W. An XPS and TPR study of the reduction of promoted cobalt-kieselguhr Fischer-Tropsch catalysts[J]. J Catal, 1986, 97(2):390-406. https://www.sciencedirect.com/science/article/pii/0021951786900114 [23] XIONG H F, ZHANG Y H, LIEW K Y, LI J L. Catalytic performance of zirconium-modified Co/Al2O3for Fischer-Tropsch synthesis[J]. J Mol Catal A:Chem, 2005, 231(1/2):145-151. http://cpfd.cnki.com.cn/Article/CPFDTOTAL-HBKJ200609003054.htm [24] CHU W, CHERNAVSKⅡ P A, GENGEMBRE L, PANKINA G A, FONGARLAND P, KHODAKOV A Y. Cobalt species in promoted cobalt alumina-supported Fischer-Tropsch catalysts[J]. J Catal, 2007, 252(2):215-230. doi: 10.1016/j.jcat.2007.09.018 [25] BEZEMER G L, BITTER J H, KUIPERS H P C E, OOSTERBEEK H, HOLEWIJN J E, XU X D, KAPTEIJN F, JOS VAN DILEN A, DE JONG K P. Cobalt particle size effects in the fischer-tropsch reaction studied with carbon nanofiber supported catalysts[J]. J Am Chem Soc, 2006, 128(12):3956-3964. doi: 10.1021/ja058282w [26] DEN BREEJEN J P, RADSTAKE P B, BEZEMER G L, BITTER J H, FRØSRTH V, HOLMEN A, DE JONG K P. On the origin of the cobalt particle size effects in Fischer-Tropsch catalysis[J]. J Am Chem Soc, 2009, 131(20):7197-7203. doi: 10.1021/ja901006x [27] JOHNSON G R, BELL A T. Role of ZrO2 in promoting the activity and selectivity of Co-based Fischer-Tropsch synthesis catalysts[J]. ACS Catal, 2016, 6(1):100-114. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=94ec6cdf6cdee2f0ddfcb3df55a7d3d2 -





 下载:
下载: