Low temperature CO oxidation over the ceria oxide catalysts doped with Fe, Ni and Cu
-
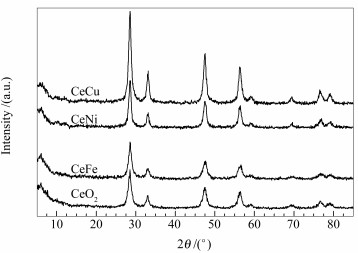
摘要: 采用水热合成法制备了一系列不同金属掺杂的Ce-M(M=Fe、Ni和Cu)复合氧化物,运用低温N2吸附-脱附、XRD、H2-TPR、拉曼光谱和XPS等表征技术对Ce-M复合氧化物的结构与其CO低温氧化反应性能之间的关系进行了关联。结果表明,将Fe、Ni和Cu掺入CeO2明显提高了其氧空位的含量,提升了晶格氧的流动性,从而使Ce-M催化剂的还原能力和催化活性高于纯CeO2。其中,CeCu催化剂氧空位最多、还原能力最好,催化活性最高,130 ℃下即可将CO完全氧化;其次是CeNi催化剂,180 ℃时实现CO完全氧化;与之相比,CeFe催化剂的活性最差,200 ℃时的CO转化率仅为92%。Abstract: A series of ceria oxide catalysts doped with Fe, Ni and Cu were prepared by hydrothermal method and they characterized by N2 sorption, XRD, H2-TPR, Raman spectra and XPS; the relationship between the structure of Ce-M mixed oxides and their catalytic performance in low temperature CO oxidation were then investigated. The results reveal that the incorporation of Fe, Ni and Cu metal ions into CeO2 can remarkably increase the amount of oxygen vacancies in the doped samples, which is beneficial to the migration of the lattice oxygen; as a result, the doped Ce-M mixed oxides exhibit much higher reducibility and catalytic activity than the pure CeO2. Among them, the CeCu catalyst with most oxygen vacancies exhibits the highest activity in CO oxidation, with a complete CO conversion at 130℃; over CeNi catalyst, in the next, a complete CO conversion is obtained at 180℃. On the contrary, CeFe catalyst is least active and the conversion of CO is only 92% at 200℃.
-
Key words:
- doped ceria /
- iron oxide /
- nickel oxide /
- copper oxide /
- oxygen vacancies /
- CO oxidation
-
表 1 Ce-M复合氧化物催化剂的ICP检测
Table 1 ICP analysis results of various Ce-M mixed oxides

表 2 CeO2及Ce-M复合氧化物催化剂的比表面积和XRD分析
Table 2 Surface area and XRD results of CeO2 and Ce-M mixed oxides with different dopants

表 3 CeO2及Ce-M复合氧化物催化剂的拉曼光谱和XPS分析
Table 3 Raman and XPS analysis results of CeO2 and Ce-M mixed oxides with different dopants

-
[1] SHAN W J, LUO M F, YING P L, SHEN W J, LI C. Reduction property and catalytic activity of Ce1-xNixO2 mixed oxide catalysts for CH4 oxidation[J]. Appl Catal A: Gen, 2003, 246 (1): 1-9. doi: 10.1016/S0926-860X(02)00659-2 [2] KIM H Y, HENKELMAN G. CO oxidation at the interface between doped CeO2 and supported Au nanoclusters[J]. J Phys Chem Lett, 2012, 3 (16): 2194-2199. doi: 10.1021/jz300631f [3] TANG C J, LI J C, YAO X J, SUN J F, CAO Y, ZHANG L, GAO F, DENG Y, DONG L. Mesoporous NiO-CeO2 catalysts for CO oxidation: Nickel content effect and mechanism aspect[J]. Appl Catal A: Gen, 2015, 494 : 77-86. doi: 10.1016/j.apcata.2015.01.037 [4] SI R. FLYTZANI-STEPHANOPOULOS M. Shape and crystal-plane effects of nanoscale ceria on the activity of Au-CeO2 catalysts for the water-gas shift reaction[J]. Angew Chem Int Ed, 2008, 47 (15): 2884-2887. doi: 10.1002/(ISSN)1521-3773 [5] 李岚, 胡庚申, 鲁继青, 罗孟飞. CeO2基固溶体氧缺位拉曼光谱表征的研究进展[J].物理化学学报, 2012, 28 (5): 1012-1020.LI Lan, HU Geng-shen, LU Ji-qing, LUO Meng-fei. Review of oxygen vacancies in CeO2-doped solid solutions as characterized by Raman spectroscopy[J]. Acta Phys-Chim Sin, 2012, 28 (5): 1012-1020. [6] LIU W, SAROFIM A F, FLYTZANI-STEPHANOPOULOS M. Reduction of sulfur dioxide by carbon monoxide to elemental sulfur over composite oxide catalysts[J]. Appl Catal B: Environ, 1994, 4 (2/3): 167-186. http://cat.inist.fr/?aModele=afficheN&cpsidt=4202677 [7] LAGUNA O H, ROMERO SARRIA F R, CENTENO M A, ODRIOZOLA J A. Gold supported on metal-doped ceria catalysts (M=Zr, Zn and Fe) for the preferential oxidation of CO (PROX)[J]. J Catal, 2010, 276 (2): 360-370. doi: 10.1016/j.jcat.2010.09.027 [8] HAN J, KIM H J, YOON S. LEE H. Shape effect of ceria in Cu/ceria catalysts for preferential CO oxidation[J]. J Mol Catal A, 2011, 335 (1/2): 82-88. https://www.deepdyve.com/lp/elsevier/shape-effect-of-ceria-in-cu-ceria-catalysts-for-preferential-co-FTdleDRwJQ [9] LI S N, ZHU H Q, QIN Z F, WANG G F, ZHANG Y G, WU Z W, LI Z K, CHEN G, DONG WW, WU Z H, ZHENG L R, ZHANG J, HU T D, WANG J G. Morphologic effects of nano CeO2-TiO2 on the performance of Au/CeO2-TiO2 catalysts in low-temperature CO oxidation[J]. Appl Catal B: Environ, 2014, 144 (2): 498-506. [10] BAO H Z, CHEN X, FANG J, JIANG Z Q, HUANG W X. Structure-activity relation of Fe2O3-CeO2 composite catalysts in CO oxidation[J]. Catal Lett, 2008, 125 (1): 160-167. [11] AVGOUROPOULOS G, IOANNIDES T, MATRALIS H. Influence of the preparation method on the performance of CuO-CeO2 catalysts for the selective oxidation of CO[J]. Appl Catal B: Environ, 2005, 56 (1/2): 87-93. http://www.sciencedirect.com/science/article/pii/S0926337304005016 [12] PAPAVASILIOU J, AVGOUROPOULOS G, IOANNIDES T. Effect of dopants on the performance of CuO-CeO2 catalysts in methanol steam reforming[J]. Appl Catal B: Environ, 2007, 69 (3/4): 226-234. http://www.sciencedirect.com/science/article/pii/S0926337306003274 [13] LI J, ZHU P F, ZHOU R X. Effect of the preparation method on the performance of CuO-MnOx-CeO2 catalysts for selective oxidation of CO in H2-rich streams[J]. J Power Sources, 2011, 196 (22): 9590-9598. doi: 10.1016/j.jpowsour.2011.07.052 [14] MAHAMMADUNNISA S, MANOJ KUMAR REDDY P, LINGAIAH N, SUBRAHMANYAM C H. NiO/Ce1-xNixO2-δ as an alternative to noble metal catalysts for CO oxidation[J]. Catal Sci Technol, 2013, 3 (3): 730-736. doi: 10.1039/C2CY20641B [15] LLIEVA L, PANTALEO G, IVANOV I, MEXIMOVA A, ZANELLA R, KASZKUR Z, VENEZIA A M, ANDREEVA D. Preferential oxidation of CO in H2 rich stream (PROX) over gold catalysts supported on doped ceria: Effect of preparation method and nature of dopant[J]. Catal Today, 2010, 158 (1/2): 44-55. http://www.sciencedirect.com/science/article/pii/S0920586110004177?via%3Dihub [16] 晏冬霞, 王华, 李孔斋, 魏永刚, 祝星, 程显名. Ce1-xFexO2复合氧化物的结构及其催化碳烟低温燃烧性能[J].物理化学学报, 2010, 26 (2): 331-337.YAN Dong-xia, WANG Hua, LI Kong-zhai, WEI Yong-gang, ZHU Xing, CHENG Xian-ming. Structure and catalytic property of Ce1-xFexO2 mixed oxide catalysts for low temperature soot combustion[J]. Acta Phys-Chim Sin, 2010, 26 (2): 331-337. [17] LIU Y M, WANG L C, CHEN M, XU J, CAO Y, HE H Y, FAN K N. Highly selective Ce-Ni-O catalysts for efficient low temperature oxidative dehydrogenation of propane[J]. Catal Lett, 2009, 130 (3): 350-354. doi: 10.1007%2Fs10562-009-9977-z/fulltext.html [18] SI R, RAITANO J, YI N, ZHANG L H, CHAN S W, FLYTZANI-STEPHANOPOULOS M. Structure sensitivity of the low-temperature water-gas shift reaction on Cu-CeO2 catalysts[J]. Catal Today, 2012, 180 (1): 68-80. doi: 10.1016/j.cattod.2011.09.008 [19] 郑修成, 张晓丽, 王淑荣, 于丽华, 王向宇, 吴世华.不同CuO/CeO2催化剂上CO低温氧化反应[J].催化学报, 2005, 26 (11): 971-976. doi: 10.3321/j.issn:0253-9837.2005.11.010ZHENG Xiu-cheng, ZHANG Xiao-li, WANG Shu-rong, YU Li-hua, WANG Xiang-yu, WU Shi-hua. Low-temperature CO oxidation over different CuO/CeO2 catalysts[J]. Chin J Catal, 2005, 26 (11): 971-976. doi: 10.3321/j.issn:0253-9837.2005.11.010 [20] FRANCISCO M S P, MASTRLARO V R, NASCENTE P A P, FLORENTINO A O. Activity and characterization by XPS, HR-TEM, Raman spectroscopy, and BET surface area of CuO/CeO2-TiO2 catalysts[J]. J Phys Chem B, 2001, 105 (43): 10515-10522. doi: 10.1021/jp0109675 [21] REDDY B M, KHAN A, YAMADA Y, KOBAYASHI T, LORIDANT S, VOLTA J C. Structural characterization of CeO2-MO2 (M = Si4+, Ti4+, and Zr4+) mixed oxides by Raman spectroscopy, X-ray photoelectron spectroscopy, and other techniques[J]. J Phys Chem B, 2003, 107 (41): 11475-11484. doi: 10.1021/jp0358376 [22] LAGUNA O H, CENTENO M A, ARZAMENDI G, GANDÍA L M, ROMERO-SARRIA F, ODRIOZOLA J A. Iron-modified ceria and Au/ceria catalysts for total and preferential oxidation of CO (TOX and PROX)[J]. Catal Today, 2010, 157 (1/4): 155-159. http://www.sciencedirect.com/science/article/pii/S0920586110002671 [23] HERNÁNDEZ W Y, CENTENO M A, ROMERO-SARRIA F, ODRIOZOLA J A. Synthesis and characterization of Ce1-xEuxO2-x/2 mixed oxides and their catalytic activities for CO oxidation[J]. J Phys Chem C, 2009, 113 (14): 5629-5635. doi: 10.1021/jp8092989 [24] QIAN K, LV S S, XIAOX Y, SUNH X, LU J Q, LUO M F, HUANG W X. Influences of CeO2 microstructures on the structure and activity of Au/CeO2/SiO2 catalysts in CO oxidation[J]. J Mol Catal A, 2009, 306 (1/2): 40-47. [25] BÊCHE E, CHARVIN P, PEREARNAU D, ABANADES S, FLAMANT G. Ce 3d XPS investigation of cerium oxides and mixed cerium oxide (CexTiyOz)[J]. Surf Interface Anal, 2008, 40 (3/4): 264-267. [26] ŚWIATOWSKA J, LAIR V, PEREIRA-NABAIS C, COTE G, MARCUS P, CHAGNES A. XPS, XRD and SEM characterization of a thin ceria layer deposited onto graphite electrode for application in lithium-ion batteries[J]. Appl Surf Sci, 2011, 257 (21): 9110-9119. doi: 10.1016/j.apsusc.2011.05.108 -





 下载:
下载: