Reaction activity and structural characterizations of sintered return fine oxyen carriers in chemical-looping combustion
-
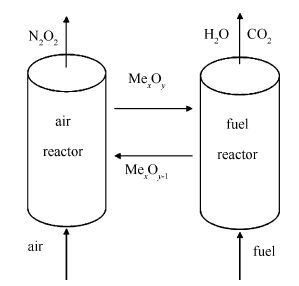
摘要: 使用流化床,以95%的甲烷作为还原性气氛,以烧结过程中烧结返矿作为载氧体,研究其在化学链燃烧过程中的反应活性以及烧结返矿在氧化还原循环中的结构特征。对烧结矿原料、还原后以及氧化再生的烧结矿进行形貌结构及物性表征。结果表明,在初始氧化循环过程中,烧结返矿的载氧能力和反应活性有显著的提高;前25个循环过程中,烧结返矿的比表面积显著增大,比表面积的增大是提高烧结返矿反应活性的主要原因之一。随着循环的进行,烧结返矿表面出现裂纹并逐渐加大。Raman检测结果显示,在还原过程中有新的铁晶型,纤铁矿(γ-FeOOH)生成,而纤铁矿(γ-FeOOH)的生成将降低烧结返矿的载氧能力。氧化循环过程中,采用甲烷作为还原性气体,并未发现有碳沉积现象的产生。Abstract: The reactivity and structural characterizations of the sintered iron ore in redox cycles were investigated. The sintered return fines were selected as oxygen carriers and the chemical looping combustion was tested in a fluidized bed with a reducing atmosphere of 95% methane. The characterizations of structural and physical properties of the sintered raw materials and the reduced and oxidative regenerated samples were conducted. The results reveal that during the initial oxidation cycle, the oxygen carrying capacity and reaction activity of the sinter are improved remarkably. The specific surface area of the sinter increases significantly in the first 25 cycles, which may be one of the main reasons for the reactivity increase of the sinter. During the recycling process, some cracks are formed on the surface of sintered return fines and gradually get development. The Raman results indicate that the new crystalline phase, lepidocrocite (γ-FeOOH), is formed, which will decrease the oxygen carrying capacity of the oxygen carrier. When the methane is used as the reducing gas, there is no carbon deposition on the surface.
-
表 1 原始烧结返矿的主要化学组成
Table 1 Chemical composition of the sintering return fines
Sample Chemical composition w/% TFe Fe2O3 CaO MgO Al2O3 SiO2 Sintering return fines 59.63 85.18 8.40 1.36 1.41 3.41 -
[1] STEWART C, HESSAMI M A.A study of methods of carbon dioxide capture and sequestration-the sustainability of a photosynthetic bioreactor approach[J].Energy Convers Manage, 2005, 46(3):403-420. doi: 10.1016/j.enconman.2004.03.009 [2] 付维新, 刘晓.天然气重整制氢及其在燃料电池的应用[J].煤气与热力, 2013, 33(6):16-21. http://www.cnki.com.cn/Article/CJFDTOTAL-MQRL201306006.htmFU Wei-xin, LIU Xiao.Hydrogen production by natural gas reforming andits application to fuel cell[J].Gas Heat, 2013, 33(6):16-21. http://www.cnki.com.cn/Article/CJFDTOTAL-MQRL201306006.htm [3] 苏俊林, 潘亮, 朱长明.富氧燃烧技术研究现状及发展[J].工业锅炉, 2008, (3):1-4. http://www.cnki.com.cn/Article/CJFDTOTAL-GYGL200803000.htmSU Jun-lin, PAN Liang, ZHU Chang-min.Research status and development of oxygen-enriched combustion technology[J].Ind Boiler, 2008, (3):1-4. http://www.cnki.com.cn/Article/CJFDTOTAL-GYGL200803000.htm [4] 毛玉如, 方梦祥, 骆仲泱, 吴学成, 岑可法.循环流化床富氧燃烧技术的试验研究[J].锅炉技术, 2004, 35(6):27-31. http://www.cnki.com.cn/Article/CJFDTOTAL-GLJS200406006.htmMAO Ru-yu, FANG Meng-xiang, LUO Zhong-yang, WU Xue-cheng, QIN Ke-fa.Experimental study on oxygen-enriched combustion technology on circulating fluidized bed test-facility[J].Boiler Technol, 2004, 35(6):27-31. http://www.cnki.com.cn/Article/CJFDTOTAL-GLJS200406006.htm [5] SAMANTA A, ZHAO A, SHIMIZU G K, SARKAR P, GUPTA R.Post-combustion CO2 capture using solid sorbents:A review[J].Ind Eng Chem Res, 2012, 51(4):1438-1463. doi: 10.1021/ie200686q [6] 周抗寒, 陆熙瑜, 艾尚坤, 刘成良.固态胺二氧化碳控制系统中的CO2浓缩技术研究[J].航天医学与医学工程, 2000, 13(3):179-182.ZHOU Kang-han, LU Xi-yu, AI Shang-kun, LIU Cheng-liang.The study on CO2 concentration in solid amine CO2 control system[J].Space Med Eng, 2000, 13(3):179-182. [7] 黄燕.疏水性沸石分子筛及其在二氧化碳控制技术中的应用[J], 应用化工, 2002, 31(3):12-15. http://www.cnki.com.cn/Article/CJFDTOTAL-SXHG200203005.htmHUANG Yan.The hydrophobic zeolite molecular sieves and its application in CO2 control technology[J].Appl Chem Ind, 2002, 31(3):12-15. http://www.cnki.com.cn/Article/CJFDTOTAL-SXHG200203005.htm [8] 沈来宏, 肖军, 肖睿, 张辉.基于CaSO4载氧体的煤化学链燃烧分离CO2研究[J], 中国电机工程学报, 2007, 27(2):69-74.SHEN Lai-hong, XIAO Jun, XIAO Rui, ZHANG Hui.Chemical looping combusiton of coalin inteconntected fluidized beds of CaSO4 oxygen carrier[J].Proc CSEE, 2007, 27(2):69-74. [9] 李振山, 韩海锦, 蔡宁生.化学链燃烧的研究现状及进展[J], 动力工程学报, 2006, 26(4):538-543.LI Zhen-shan, HAN Hai-mian, CAI Ning-sheng.Research status and progress of chemical-looping combustion[J].J Chin Soc Power Eng, 2006, 26(4):538-543. [10] 金红光, 洪慧, 韩涛.化学链燃烧的能源环境系统研究进展[J], 科学通报, 2008, 53(24):2994-3005. http://www.cnki.com.cn/Article/CJFDTOTAL-KXTB200824003.htmJIN Hong-guang, HONG Hui, HAN Tao.Research progress on energy environment system of chemical-looping combustion[J].Chin Sci Bull, 2008, 53(24):2994-3005. http://www.cnki.com.cn/Article/CJFDTOTAL-KXTB200824003.htm [11] MERKEL T C, LIN H, WEI X, BAKER R.Power plant post-combustion carbon dioxide capture:An opportunity for membranes[J].J Membr Sci, 2010, 359(1):126-139. [12] KANNICHE M, GROS B R, JAUD P, VALLE M J, AMANN J M, BOUALLOU C.Pre-combustion, post-combustion and oxy-combustion in thermal power plant for CO2 capture[J].Appl Therm Eng, 2010, 30(1):53-62. doi: 10.1016/j.applthermaleng.2009.05.005 [13] SCHOLES C A, SMITH K H, KENTISH S E, STEVENS G W.CO2 capture from pre-combustion processes-strategies for membrane gas separation[J].Int J Greenh Gas Con, 2010, 4(5):739-755. doi: 10.1016/j.ijggc.2010.04.001 [14] 杨明明, 刘永卓, 贾伟华, 胡修德, 郭庆杰.Fe2O3/ATP载氧体制备及煤化学链燃烧性能研究[J].燃料化学学报, 2015, 43(2):167-176. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18571.shtmlYANG Ming-ming, LIU Yong-zhuo, JIA Wei-hua, HU Xiu-de, GUO Qing-jie.Preparation and performance of the Fe2O3/ATP oxygen carriers in coal chemical looping combustion[J].J Fuel Chem Technol, 2015, 43(2):167-176. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18571.shtml [15] 陈定千, 沈来宏, 肖军, 宋涛, 顾海明, 张思文.基于镍基修饰的铁矿石载氧体煤化学链燃烧实验[J].燃料化学学报, 2012, 40(3):267-272. doi: 10.1016/S1872-5813(12)60015-2CHEN Ding-qian, SHEN Lai-hong, XIAO Jun, SONG Tao, GU Hai-ming, ZHANG Si-wen.Experimental investigation of hematite oxygen carrier decorated with NiO for chemical looping combustion of coal[J].J Fuel Chem Technol, 2012, 40(3):267-272. doi: 10.1016/S1872-5813(12)60015-2 [16] SARSHAR Z, KLEITZ F, KALIAGUINE S.Novel oxygen carriers for chemical looping combustion:La1-xCexBO3(B=Co, Mn) perovskites synthesized by reactive grinding and nanocasting[J].Energy Environ Sci, 2011, 4(10):4258-4269. doi: 10.1039/c1ee01716k [17] PENTHOR S, MAYER K, KERN S, KITZLER H, WÖSS D, PRÖLL T, HOFBAUER H.Chemical-looping combustion of raw syngas from biomass steam gasification-Coupled operation of two dual fluidized bed pilot plants[J].Fuel, 2014, 127:178-185. doi: 10.1016/j.fuel.2014.01.062 [18] ABAD A, ADÁNEZ J, CUADRAT A, GARCÍA L F, GAYÁN P, DE DIEGO L F.Kinetics of redox reactions of ilmenite for chemical-looping combustion[J].Chem Eng Sci, 2011, 66(4):689-702. doi: 10.1016/j.ces.2010.11.010 [19] LEION H, MATTISSON T, LYNGFELT A.Use of ores and industrial products as oxygen carriers in chemical-looping combustion[J].Energy Fuels, 2009, 23(4):2307-2315. doi: 10.1021/ef8008629 [20] XI G, WANG C, WANG X.The oriented self-assembly of magnetic Fe3O4 nanoparticles into monodisperse microspheres and their use as substrates in the formation of Fe3O4 nanorods[J].Eur J Inorg Chem, 2008, (3):425-431. [21] SHEBANOVA O N, LAZOR P.Raman spectroscopic study of magnetite (Fe3O4):A new assignment for the vibrational spectrum[J].J Solid State Chem, 2003, 174(2):424-430. doi: 10.1016/S0022-4596(03)00294-9 [22] THIBEAU R J, BROWN C W, HEIDERSBACH R H.Raman spectra of possible corrosion products of iron[J].Appl Spectrosc, 1978, 32(6):532-535. doi: 10.1366/000370278774330739 [23] DE-FARIA D, VENÂNCIO S S, DE-OLIVEIRA M.Raman microspectroscopy of some iron oxides and oxyhydroxides[J].J Raman Spectrosc, 1997, 28(11):873-878. doi: 10.1002/(ISSN)1097-4555 [24] LI Y S, CHURCH J S, WOODHEAD A L.Infrared and Raman spectroscopic studies on iron oxide magnetic nano-particles and their surface modifications[J].J Magn Mater, 2012, 324(8):1543-1550. doi: 10.1016/j.jmmm.2011.11.065 [25] SHEBANOVA O N, LAZOR P.Raman study of magnetite (Fe3O4):laser-induced thermal effects and oxidation[J].J Raman Spectrosc, 2003, 34(11):845-852. doi: 10.1002/(ISSN)1097-4555 [26] PIMENTA H P, SESHADRI V.Characterisation of structure of iron ore sinter and its behaviour during reduction at low temperatures[J].Ironmak Steelmak, 2002, 29(3):169-174. doi: 10.1179/030192302225002009 [27] CUI X, ANTONIETTI M, YU S H.Structural effects of iron oxide nanoparticles and iron ions on the hydrothermal carbonization of starch and rice carbohydrates[J].Small, 2006, 2(6):756-759. doi: 10.1002/(ISSN)1613-6829 [28] TAY H L, LI C Z.Changes in char reactivity and structure during the gasification of a Victorian brown coal:Comparison between gasification in O2 and CO2[J].Fuel Process Technol, 2010, 91(8):800-804. doi: 10.1016/j.fuproc.2009.10.016 -





 下载:
下载: