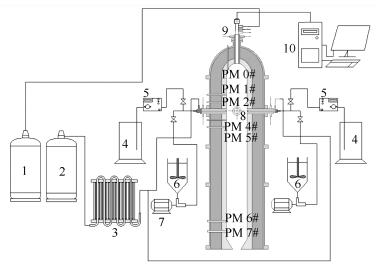
Release and transformation of sodium in an opposed multi-burner coal-water slurry gasifier
-
摘要: 基于多喷嘴对置式水煤浆气化热态实验平台,开展气化炉内钠元素的释放与转化特性研究。对收集的气化炉内轴向不同位置处颗粒物样品进行分析,利用微波消解和化学分级洗对颗粒物进行预处理,借助原子火焰吸收光谱仪(FASS)定量测定颗粒物中钠元素含量,并采用扫描电镜-能谱仪联用系统(SEM-EDS)表征颗粒物的表观形态及其表面元素组成。FAAS测定结果表明,钠元素释放率随距离喷嘴平面间距的增加呈先增加再减少的趋势,喷嘴平面附近为钠元素主要释放区域。随着炉内反应的进行,钠元素由水溶态、离子交换态向酸溶态和残渣态转变。结合SEM-EDS结果可知,炉内反应过程中,矿物质熔融形成球形颗粒物,与气相中的钠反应生成硅酸盐和硅铝酸盐,且钠元素含量随球形颗粒物的增多而增加。Abstract: Based on the bench-scale opposed multi-burner (OMB) coal-water slurry gasification experimental platform, the transformation and release characteristics of sodium during the reaction of coal in the gasifier were studied. Particles sampled at different axial distances from burner plane to top and bottom along the gasifier chamber were analyzed. After the microwave digestion and chemical fractionation analysis, the content of sodium was tested by flame atomic absorption spectrometer (FASS). The morphololgy and elements of particles were analyzed by scanning electron microscopy and energy spectrum application system (SEM-EDS). The FASS results showed that the release rate of sodium increased first then decreased with increasing distance to the burner plane. The area near the burner plane was the major release area of sodium. With the reaction in progress in the gasifier the occurrence form of sodium was transformed from water-soluble sodium and ion-exchangeable sodium into the acid-soluble sodium and residual sodium. Combining the SEM-EDS and FASS results, the spherical particles which were formed through melt minerals reacted with sodium in the gas phase to form silicate and sialic acid salt in gasifier. The increasing number of spherical particles led to an increase in the sodium content in the particles.
-
Key words:
- opposed multi-burner (OMB) gasifier /
- particles /
- sodium /
- release /
- occurrence form
-
表 1 煤样的工业分析和元素分析
Table 1 Proximate and ultimate analyses of coal
Proximate analysis wad/% Ultimate analysis wad/% M A V FC C H O N S 1.38 10.59 32.19 55.84 71.8 4.61 11.07 1.17 0.75 表 2 煤样的灰分组成
Table 2 Chemical composition of coal ash
Composition w/% SiO2 Al2O3 Fe2O3 Na2O CaO SO3 TiO2 MgO K2O P2O5 others 49.01 32.48 5.31 3.97 3.64 2.03 1.23 0.73 0.72 0.63 0.25 表 3 颗粒物中不同赋存形态钠的含量
Table 3 Sodium content of different occurrence forms in coal
H2O NH4AC HCl Residue Total Content w/ (mg·g-1) 2.48 0.55 0.31 0.86 4.20 Percent w/% 59.04 13.09 7.32 20.50 100.00 表 4 不同区域元素的组成
Table 4 Element distribution in different area
Content w/% C O Na Mg Al Si S Fe Ca others a-1 35.66 44.25 2.62 0.81 7.06 7.76 0.15 0.35 0.81 0.53 a-2 72.79 22.29 1.35 0.31 1.49 1.24 0.18 0.06 0.06 0.61 a-3 64.35 32.56 0.82 0.06 0.18 0.15 0.37 0.46 0.16 0.89 b-1 31.59 46.47 3.54 0.77 9.23 6.53 0.22 0.43 0.48 0.75 b-2 79.23 16.11 0.81 0.20 1.35 1.33 0.54 0.04 0.05 0.34 b-3 83.74 13.22 0.66 0.16 0.47 1.14 0.32 0.02 0.01 0.26 c-1 29.13 49.02 3.96 0.85 7.93 7.50 0.17 0.27 0.56 0.60 c-2 77.04 15.29 0.84 0.14 3.32 2.66 0.28 0.02 0.02 0.40 c-3 83.40 13.98 0.81 0.18 0.58 0.54 0.16 0.04 0.03 0.27 d-1 19.62 60.75 4.79 0.56 10.62 3.07 0.17 0.27 0.12 0.03 d-2 36.98 2.75 0.00 0.01 14.93 23.73 0.36 8.70 8.40 4.15 -
[1] 于广锁, 牛苗任, 王亦飞, 梁钦锋, 于遵宏.气流床煤气化的技术现状和发展趋势[J].现代化工, 2004, 24(5):23-26. http://www.cnki.com.cn/Article/CJFDTotal-XDHG200405007.htmYU Guang-suo, NIU Miao-ren, WANG Yi-fei, LIANG Qin-feng, YU Zun-hong. Application status and development tendency of coal entrained-bed gasification[J]. Mod Chem Ind, 2004, 24(5):23-26. http://www.cnki.com.cn/Article/CJFDTotal-XDHG200405007.htm [2] 王辅臣.气流床煤气化炉内流动、混合与反应过程的研究进展[J].燃料化学学报, 2013, 41(7):769-786. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18210.shtmlWANG Fu-chen. Review for research of flow, mixing and reaction process in entrained flow coal gasifier[J]. J Fuel Chem Technol, 2013, 41(7):769-786. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18210.shtml [3] 王辅臣, 于广锁, 龚欣, 刘海峰, 王亦飞, 梁钦峰.大型煤气化技术的研究与发展[J].化工进展, 2009, 28(2):173-180.WANG Fu-chen, YU Guang-suo, GONG Xin, LIU Hai-feng, WANG Yi-fei, LIANG Qin-feng. Research and development of large-scale coal gasification technology[J]. Chem Ind Eng Prog, 2009, 28(2):173-180. [4] 隋建才, 徐明厚, 丘纪华, 郭欣, 刘小伟, 高翔鹏.燃煤锅炉PM (10)形成与排放特性的实验研究[J].中国工程热物理学报, 2006, 27(2):335-338. http://www.cnki.com.cn/Article/CJFDTotal-GCRB200602050.htmSUI Jian-cai, XU Ming-hou, QIU Ji-hua, GUO Xin, LIU Xiao-wei, GAO Xiang-peng. The experimental study of PM (10) formation and emission characteristics in coal-fired boiler[J]. J Eng Thermophys, 2006, 27(2):335-338. http://www.cnki.com.cn/Article/CJFDTotal-GCRB200602050.htm [5] LOCKWOOD F C, YOUSIF S. A model for the particulate matter enrichment with toxicmetals in solid fuel flames[J]. Fuel Process Technol, 2000, 65:439-457. [6] SENIOR C L, HELBLE J J, SAROFIM A F. Emissions of mercury, trace elements, and fine particles from stationary combustion sources[J]. Fuel Process Technol, 2000, 65-66:263-288. doi: 10.1016/S0378-3820(00)00082-5 [7] 宋维健, 宋国良, 张海霞, 范金龙, 吕清刚.准东高钠煤热解过程中钠的迁移特性实验研究[J].燃料化学学报, 2015, 43(1):16-21. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18549.shtmlSONG Wei-jian, SONG Guo-liang, ZHANG Hai-xia, FAN Jin-long, LÜ Qing-gang. Experimental study on alkali metal transformation during high-sodium Zhundong coal pyrolysis[J]. J Fuel Chem Technol, 2015, 43(1):16-21. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18549.shtml [8] 宋维健, 宋国良, 齐晓宾, 吕清刚.准东高钠煤气化过程中Na的迁移转化规律[J].煤炭学报, 2016, (2):490-496. http://www.cnki.com.cn/Article/CJFDTotal-MTXB201602030.htmSONG Wei-jian, SONG Guo-liang, QI Xiao-bin, LÜ Qing-gang. Experimental study on sodium transformation during high-sodium Zhundong coal gasification[J]. J China Coal Soc, 2016, (2):490-496. http://www.cnki.com.cn/Article/CJFDTotal-MTXB201602030.htm [9] 卫小芳, 刘铁峰, 黄戒介, 房倚天, 王洋.澳大利亚高盐煤中钠在热解过程中的形态变迁[J].燃料化学学报, 2010, 38(2):144-148. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract17553.shtmlWEI Xiao-fang, LIU Tie-feng, HUANG Jie-jie, FANG Yi-tian, WANG Yang. Transformation of Na in an Australian high-sodium coal during pyrolysis[J]. J Fuel Chem Technol, 2010, 38(2):144-148. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract17553.shtml [10] DAI B Q, WU X, DE GIROLAMO A, ZHANG L. Inhibition of lignite ash slagging and fouling upon the use of a silica-based additive in an industrial pulverised coal-fired boiler:Part 1. Changes on the properties of ash deposits along the furnace[J]. Fuel, 2015, 139:720-732. doi: 10.1016/j.fuel.2014.06.054 [11] DAI B Q, WU X, DE GIROLAMO A, ZHANG L. Inhibition of lignite ash slagging and fouling upon the use of a silica-based additive in an industrial pulverised coal-fired boiler:Part 2. Speciation of iron in ash deposits and separation of magnetite and ferrite[J]. Fuel, 2015, 139:733-745. doi: 10.1016/j.fuel.2014.06.075 [12] LOW F, DE GIROLAMO A, WU X, DAI B Q, ZHANG L. Inhibition of lignite ash slagging and fouling upon the use of a silica-based additive in an industrial pulverised coal-fired boiler:Part 3. Partitioning of trace elements[J]. Fuel, 2015, 139:746-756. doi: 10.1016/j.fuel.2014.09.015 [13] WANG C, XI J, WANG Y, WANG Y K, YAN Y, JIANG C, LIU Y H, CHE D F. Release and transformation of sodium during pyrolysis of Zhundong coals[J]. Energy Fuels, 2015, 29(1):48-56. [14] LI G Y, WANG C A, YAN Y, XI J, LIU Y H, CHE D F. Release and transformation of sodium during combustion of Zhundong coals[J]. J Energy Inst, 2015, 29(1):48-56. [15] CHEN H D, CHEN X L, ZHI Q, LIU H F. Release and transformation characteristics of K and Cl during straw torrefaction and mild pyrolysis[J]. Fuel, 2016, 167:31-39. doi: 10.1016/j.fuel.2015.11.059 [16] BENSON S A, HOLM P L. Comparison of inorganic constituents in three low-rank coals[J]. Ind Eng Chem Prod Res Dev, 1985, 24(1):145-149. doi: 10.1021/i300017a027 [17] SHUANGNING X, ZHIHE L, BAOMING L. Devolatilization characteristics of biomass at flash heating rate[J]. Fuel, 2006, 85(5):664-670. [18] MENG F, YU J, TAHMASEBI A, HAN Y. Pyrolysis and combustion behavior of coal gangue in O2/CO2 and O2/N2 mixtures using thermogravimetric analysis and a drop tube furnace[J]. Energy Fuels, 2013, 27(6):2923-2932. doi: 10.1021/ef400411w [19] KOSMINSKI A, ROSS D P, AGNEW J B. Reactions between sodium and silica during gasification of a low-rank coal[J]. Fuel Process Technol, 2006, 87(12):1037-1049. doi: 10.1016/j.fuproc.2005.06.007 [20] QUYN D M, WU H, BHATTACHARYA S P, LI C Z. Volatilisation and catalytic effects of alkali and alkaline earth metallic species during the pyrolysis and gasification of Victorian brown coal. Part Ⅱ. Effects of chemical form and valence[J]. Fuel, 2002, 81(2):151-158. doi: 10.1016/S0016-2361(01)00128-4 [21] WEI X, HUANG J, LIU T, WANG Y. Transformation of alkali metals during pyrolysis and gasification of a lignite[J]. Energy Fuels, 2008, 22(3):1840-1844. doi: 10.1021/ef7007858 [22] WOLF K J, MVLLER M, HILPERT A K, SINGHEISER L. Alkali sorption in second-generation pressurized fluidized-bed combustion[J]. Energy Fuels, 2004, 18(6):1841-1850. doi: 10.1021/ef040009c [23] WALL C J, GRAVES J T, ROBERTS E J. How to burn salty sludges[J]. Chem Eng, 1975, 82(8):77-82. [24] 孙利军, 龚岩, 郭庆华, 于广锁.多喷嘴对置式水煤浆气化炉内固体颗粒微观特性研究[J].燃料化学学报, 2014, 42(9):1025-1032. doi: 10.1016/S1872-5813(14)60042-6SUN Li-jun, GONG Yan, GUO Qing-hua, YU Guang-suo. Microscopic characteristics of solid particles in opposed multi-burner gasifier[J]. J Fuel Chem Technol, 2014, 42(9):1025-1032. doi: 10.1016/S1872-5813(14)60042-6 [25] 李文, 白进.煤的灰化学[M].北京:科学出版社, 2013.LI Wen, BAI Jing. Chemisty of Ash form Coal[M]. Beijing:Science Press, 2013. -





 下载:
下载: