Thermodynamic study on effect of minerals in fly ash on morphological distribution of As, Se and Pb in flue gas
-
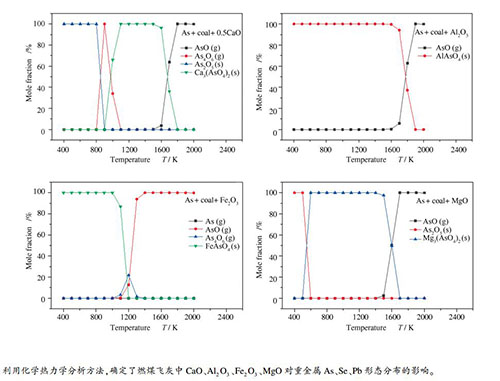
摘要: 基于热力学平衡的原理,研究了燃煤烟气中As、Se、Pb三种重金属与飞灰中主要矿物质CaO、Al2O3、Fe2O3、MgO的反应。结果表明,As在1600 K时与CaO反应开始生成Ca3(AsO4)2,且随着CaO含量的增大,Ca3(AsO4)2的温度区间变窄,说明CaO对煤中As的挥发有抑制作用;As与Al2O3在1700 K时开始反应,随着温度降低最后全部以AlAsO4的形式存在;As与Fe2O3反应生成FeAsO4;As与MgO在600-1500 K以Mg3(AsO4)2(s)形式存在,低于600 K时转化为As2O5(s)。Se与CaO和MgO在低于600 K时分别以CaSeO3(s)和MgSeO3(s)的形式存在,与Al2O3、Fe2O3不发生反应;CaO与Pb在900-1100 K时反应生成了(CaO)2(PbO2)(s);Pb与Al2O3会发生反应,在900-1200 K时有固态(PbO)(Al2O3)6(s)生成。Fe2O3、MgO对Pb的形态分布基本没有影响。Abstract: Based on the principle of thermodynamic equilibrium, reactions between heavy metals As, Se and Pb in flue gas of coal burning and main minerals CaO, Al2O3, Fe2O3 and MgO in fly ash were studied. The results show that As reacts with CaO at 1600 K to form Ca3(AsO4)2, and its temperature range becomes narrower with increasing CaO concentration, indicating that CaO can inhibit volatilization of As in coal. As reacts with Al2O3 at 1700 K, reaction of As with Fe2O3 forms FeAsO4. As and MgO exist in the form of Mg3(AsO4)2(s) between 600 and 1500 K, and turns into As2O5(s) below 600 K. Se and CaO, MgO exist in the form of CaSeO3(s) and MgSeO3(s), respectively, below 600 K, but does not react with Al2O3 and Fe2O3. CaO and Pb react at 900-1100 K to form (CaO)2(PbO2)(s). Pb reacts with Al2O3, and solid (PbO)(Al2O3)6(s) is formed at 900-1200 K. Fe2O3 and MgO have no effect on species distribution of Pb.
-
Key words:
- minerals /
- coal-fired flue gas /
- heavy metals /
- morphological distribution
-
表 1 输入煤初始条件
Table 1 Initial condition of coal input
Element C H O N S Cl As Se Pb Quantity/mol 64.04 37.5 176.35 659.2 0.178 0.845×10-3 3.19×10-3 7.06×10-3 5.22×10-3 Content 76.85% 3.75% 2.20% 1.37% 0.57% 30 μg/g 2.39 μg/g 5.58 μg/g 10.81 μg/g 表 2 煤样灰分
Table 2 Ash of coal sample
Mineral Al2O3 CaO Fe2O3 K2O Na2O MgO TiO2 SiO2 Quantity/mol 0.4765 0.1297 0.0355 0.0126 0.0162 0.0393 0.0269 1.2387 Content/% 33.46 5.01 3.91 0.82 0.69 1.09 1.48 51.26 -
[1] 李昌鑫, 王昊, 叶坚锴, 肖小芹, 王舒文, 何云峰.燃煤电厂区域颗粒物及颗粒物汞分布特征研究[J].环境科学学报, 2020, 40(8): 2944-2951.LI Chang-xin, WANG Hao, YE Jian-kai, XIAO Xiao-qin, WANG Shu-wen, HE Yun-feng. Pollution characteristics of particulate matter and particulate mercury near a coal-fired power plant[J]. Acta Sci Circumstantiae, 2020, 40(8): 2944-2951. [2] 梁斌, 白浩隆, 冯强, 宋华, 蓝天, 刘新华.民用燃煤颗粒物及多环芳烃排放特性[J].化工学报, 2019, 70(8): 2888-2897+3212.LIANG Bin, BAI Hao-long, FENG Qiang, SONG Hua, LAN Tian, LIU Xin-hua. Emissions of particulate matter and polycyclic aromatic hydrocarbons from household coal combustions[J]. J Chem Ind Eng, 2019, 70(8): 2888-2897+3212. [3] 乔岗杰, 刘轩, 赵元财, 刘红刚, 孔凡荣, 张锴.燃煤电厂典型重金属排放与控制进展[J].电站系统工程, 2020, 36(2): 1-4+8.QIAO Gang-jie, LIU Xuan, ZHAO Yuan-cai, LIU Hong-gang, KONG Fan-rong, ZAHNG Kai. Emission of typical heavy metal from coal-fired power plants and control[J]. Power Syst Eng, 2020, 36(2): 1-4+8. [4] 李云, 郭伟.煤的工业分析指标和指标关系剖析[J].科技与创新, 2014, (6): 155.LI Yun, GUO Wei. Analysis of indicators and indicators of industrial relations coal analysis[J]. Sci Technol Innov, 2014, (6): 155. [5] 郭富强, 刘清才, 蒋历俊, 任山, 王铸, 赵齐.粒径和燃烧温度对燃煤颗粒物微观形貌影响研究[C].中国环境科学学会, 2016: 61-65.GUO Fu-qiang, LIU Qing-cai, JIANG Li-jun, REN Shan, WANG Zhu, Zhao Qi. Influence of burning temperature and particle size on particulate matter microstructure during pulverized coal combustion[C]. Chin Soc Environ Sci, 2016: 61-65. [6] 邓双, 张凡, 刘宇, 石应杰, 王红梅, 张辰, 王相凤, 曹晴.燃煤电厂铅的迁移转化研究[J].中国环境科学, 2013, 33(7): 1199-1206.DENG Shuang, ZHANG Fan, LIU Yu, SHI Ying-jie, WANG Hong-mei, ZHANG Chen, WANG Xiang-feng, CAO Qing. Lead emission and speciation of coal-fired power plants in China[J]. Chin Environ Sci, 2013, 33(7): 1199-1206. [7] 马杨杨, 仲兆平, 赖旭东.矿物添加剂对煤燃烧过程中重金属的富集[J].化工进展, 2020, 39(6): 2479-2486.MA Yang-yang, ZHONG Zhao-ping, LAI Xu-dong. Enrichment of heavy metals during coal combustion by mineral additives[J]. Chem Ind Eng Prog, 2020, 39(6): 2479-2486. [8] ZHA J, HUANG Y, XIA W, XIA Z, LIU C, DONG L, LIU L. Effect of mineral reaction between calcium and aluminosilicate on heavy metal behavior during sludge incineration[J]. Fuel, 2018, 229: 241-247. [9] WANG C, LIU H, ZHANG Y, ZOU C, EDWARD J. Review of arsenic behavior during coal combustion: Volatilization, transformation, emission and removal technologies[J]. Energy Combust, 2018, 68(S): 1-28. [10] 郭胜利.燃煤重金属迁移转化特性及其污染控制研究[D].重庆: 重庆大学, 2014.GUO Sheng-li. Research on the characteristic of movement and transformation and pollution control of heavy metals in coal combustion[D]. Chongqing: Chongqing University, 2014. [11] LEI M, DONG Z, JIANG Y, LONGHURST P, WAN X, ZHOU G. Reaction mechanism of arsenic capture by a calcium-based sorbent during the combustion of arsenic-contaminated biomass: A pilot-scale experience[J]. Front Environ Sci Eng, 2019, 13(2): P.105-113. [12] 张淑会, 吕庆, 胡晓.吸附剂烟气脱砷的研究现状[J].环境科学与技术, 2011, 34(3): 197-204.ZHANG Shu-hui, LV Qing, HU Xiao. Review on sorbents for arsenic removal from flue gas[J]. Environ Sci Technol, 2011, 34(3): 197-204. [13] 陈敦奎.燃烧烟气中砷的吸附控制机理研究[D].武汉: 华中科技大学, 2016.CHEN Dun-kui. Investigation on the Adsorption Mechanism of Arsenic in Flue Gas during Fuel Combustion. Wuhan: Huazhong University of Science and Technology, 2016. [14] YANG Y, HU H, XIE K, HUANG Y, LIU H, LI X, YAO H, NARUSE I. Insight of arsenic transformation behavior during high-arsenic coal combustion[J]. Proc Combust Inst, 2019, 37(4): 4443-4450. [15] ZHANG K, WANG P, ZHANG D, ZHANG K. Studies on the correlation between physicochemical properties of fly ash and its sorption of gas-phase arsenic[J]. Environ Technol, 2019, 40(19): 2548-2555. [16] ZHOU C, LIU G, XU Z, SUN H, SING LAM P K. Effect of ash composition on the partitioning of arsenic during fluidized bed combustion[J]. Fuel, 2017, 204(s): 91-97. [17] 张月, 王春波, 刘慧敏, 孙喆, 李文瀚, 张永生, 潘伟平.金属氧化物吸附剂干法脱除气相As2O3实验研究[J].燃料化学学报, 2015, 43(4): 476-482.ZHANG Yue, WANG Chun-bo, LIU Hui-min, SUN Zhe, LI Wen-han, ZHANG Yong-sheng, PAN Wei-ping. Removal of gas-phase As 2 O 3 in dry process by metal oxide adsorbents[J]. J Fuel Chem Technol, 2015, 43(4): 476-482. [18] 李玉忠.中温脱硫过程联合脱除痕量硒、砷的实验研究[D].北京: 清华大学, 2006.LI Yu-zhong. Experimental study on simultaneous removal of trace selenium and arsenic in flue gas desulphurization within medium temperature range[D]. Beijing: Tsinghua University, 2006. [19] 张军营, 任德贻, 钟秦, 徐复铭, 张衍国. CaO对煤中砷挥发性的抑制作用[J].燃料化学学报, 2000, 28(3): 198-200.ZHANG Jun-ying, REN De-yi, ZHONG Qin, XU Fu-ming, ZHANG Yan-guo. Restraining of arsenic volatility using lime in coal combustion[J]. J Fuel Chem Technol, 2000, 28(3): 198-200. [20] FOLGUERAS M B, MARÍA D R, XIBERTA J, ALONSO M. Effect of Inorganic Matter on Trace Element Behavior during Combustion of CoalSewage Sludge Blends[J]. Energy Fuels, 2007, 21(2): 744-755. [21] JIAO F, ZHANG L, SONG W. Effect of inorganic particulates on the condensation behavior of lead and zinc vapors upon flue gas cooling[J]. Proceedings of the Combustion Institute, 2013, 34(2): 2821-2829. [22] 孟韵, 张军营, 钟秦, 郑楚光, 王犇.燃煤过程中微量元素砷和硒形态转化的热力学平衡模拟[J].环境污染治理技术与设备, 2002, (9): 1-5.MENG Yun, ZHANG Jun-ying, ZHONG Qin, ZHENG Chu-guang, WANG Ben. Development of thermodynamic equilibrium prediction of trace arsenic and selenium speciation during coal combustion[J]. Techn Equi Environ Pollut Control, 2002, (9): 1-5. [23] 张璐.化学链燃烧技术中氧载体CaSO <, 4>的性能研究[D].武汉: 华中科技大学, 2009.ZHANG Lu. Study on the performance of oxygen carrier CaSO <, 4> in chemical chain combustion technology[D]. Wuhan: Huazhong University of Science and Technology, 2009. [24] 范巍.不锈钢厂烟尘的理化特性及其中含铬物相形成[D].武汉: 武汉科技大学, 2012.FAN Wei. Physical and chemical properties of smoke and dust in stainless steel factory and formation of chromium-containing phase[D]. Wuhan: Wuhan University of Science and Technology, 2012. [25] CHEN D, HU H, XU Z, LIU H, CAO J, SHEN J, YAO H. Findings of proper temperatures for arsenic capture by CaO in the simulated flue gas with and without SO2[J]. Chem Eng J, 2015, 267: 201-206. -





 下载:
下载: