Performance of manganese-zirconium composite oxide in the catalytic reduction of NO by CO
-
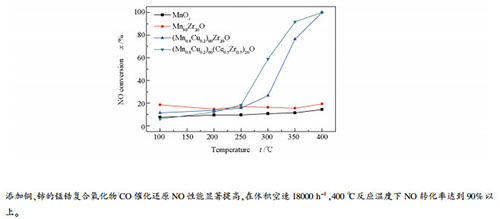
摘要: 采用柠檬酸络合法制备了锰锆复合氧化物催化剂,用XRD、H2-TPR、XPS和SEM等技术进行了表征,研究了其CO催化还原NO性能。结果表明,MnOx主要以Mn3O4物相存在,Zr占比的增加会促进Mn3O4物相的分散,引起Mn3O4平均晶粒粒径减小;Mn主要有Mn2+、Mn3+、Mn4+离子价态形式,添加Cu和Ce后,(Mn3++Mn4+)含量和表面吸附氧(OA)含量增加,H2-TPR还原峰温度向低温区偏移,有利于催化活性的提升。Mn-Zr-O复合氧化物的CO催化还原NO活性较低,加入Cu后的Mn-Cu-Zr-O复合氧化物其CO催化还原NO的活性得到改善,而添加Ce后所制备的Mn-Cu-Ce-Zr-O复合氧化物的催化活性进一步提高;在350 ℃下、反应空速为18000 h-1时,Mn-Cu-Ce-Zr-O复合氧化物表现出较好的CO催化还原NO活性,CO转化率达到了89.17%,NO转化率达到了91.70%。Abstract: A series of manganese-zirconium composite oxides were prepared by citric acid complexing method and characterized by XRD, H2-TPR, XPS and SEM; their performance in the catalytic reduction of NO by CO was investigated. The results show that Mn3O4 is the main phase for MnOx in the Mn-Zr composite oxide; an increase in the Zr content can promote the dispersion of Mn3O4 and reduce the average grain size of Mn3O4. Mn may exist in the form of Mn2+, Mn3+ and Mn4+ ions; the content of (Mn3+ + Mn4+) and the quantity of surface adsorbed oxygen (OA) increase after the addition of Cu and Ce, which is beneficial to enhancing the catalytic activity. The pristine Mn-Zr-O composite shows a relatively low activity in the catalytic reduction of NO by CO; after adding Cu, the Mn-Cu-Zr-O composite exhibits much higher activity than Mn-Zr-O; moreover, the activity of Mn-Cu-Ce-Zr-O composite is even enhanced by adding Ce. For the catalytic reduction of NO by CO over the Mn-Cu-Ce-Zr-O composite at 350℃ and with a space velocity of 18000 h-1, the CO conversion and NO conversion are 89.17% and 91.70%, respectively.
-
Key words:
- Mn-based composite oxide /
- CO /
- NO /
- catalytic reduction /
- activity
-
表 1 Mn-Zr-O复合氧化物Mn3O4物相晶胞参数及晶胞体积
Table 1 Crystal cell parameters and unit cell volume of Mn3O4 phase of various Mn-Zr-O composites
Catalyst 2θ/(°) Mn3O4 103 211 a/nm b/nm c/nm v0/nm3 MnOx 32.3349 36.1099 0.5764 0.5764 0.9470 0.3146 Mn80Zr20O 32.3259 36.0719 0.5771 0.5771 0.9470 0.3153 (Mn0.8Cu0.2)80Zr20O 32.4159 36.0499 0.5776 0.5776 0.9434 0.3147 (Mn0.8Cu0.2)80(Ce0.5Zr0.5)20O 32.4649 36.0519 0.5776 0.5776 0.9416 0.3142 表 2 复合氧化物Mn3O4物相平均晶粒粒径
Table 2 Average grain size of Mn3O4 phase of various Mn-Zr-O composites
Catalyst Mn3O4(103) 2θ/(°) full width at half maximum(β) average crystallite size d/nm MnOx 32.3349 0.223 92.19 Mn80Zr20O 32.3259 0.738 11.98 (Mn0.8Cu0.2)80Zr20O 32.4159 0.760 11.60 (Mn0.8Cu0.2)80(Ce0.5Zr0.5)20O 35.8709 0.966 9.30 表 3 催化剂Mn 2p、O 1s峰曲线拟合结果
Table 3 Curve fitting results of Mn 2p and O 1s XPS spectra
Catalyst Ratio/% Mn2+/(Mn2++Mn3++Mn4+) (Mn3++Mn4+)/(Mn2++Mn3++Mn4+) OA/(OA+OL) Mn-Zr-O 46.87 53.13 31.58 Mn-Cu-Zr-O 43.19 56.81 34.66 Mn-Cu-Ce-Zr-O 35.94 64.06 48.27 -
[1] TRINH H T, IMANISHI K, MORIKAWAI T, HAGINO H, TAKENAKA N. Gaseous nitrous acid (HONO) and nitrogen oxides (NOx) emission from gasoline and diesel vehicles under real-world driving test cycles[J]. Air Repair, 2017, 67(4): 412-420. [2] DIAO B D, DING L, SU P D, CHENG J H. The spatial-temporal characteristics and influential factors of NOx emissions in China: A spatial econometric analysis[J]. Int J Environ Res Public Health, 2018, 15(7): 1405. doi: 10.3390/ijerph15071405 [3] GE X Y, ZHOU Z M, ZHOU Y L, YE X Y, LIU S L. A spatial panel data analysis of economic growth, urbanization, and NOx emissions in China[J]. Int J Environ Res Public Health, 2018, 15(4): 725. doi: 10.3390/ijerph15040725 [4] TIBOR B, MILAN V, HRVOJE M, NEVEN D. Numerical modelling of emissions of nitrogen oxides in solid fuel combustion[J]. J Environ Manage, 2018, 215: 177-184. doi: 10.1016/j.jenvman.2018.03.014 [5] LI J H, PENG Y, CHANG H Z, LI X, RITTENDEN J C, HAO J M. Chemical poison and regeneration of SCR catalysts for NOx removal from stationary sources[J]. Front Environ Sci Eng, 2016, 10(3): 413-427. doi: 10.1007/s11783-016-0832-3 [6] LIU K, YAO Y Y, WEI L Q, SHI Z F. Preparation and evaluation of Cu-Mn-oxide as high efficiency catalyst for CO oxidation and NO reduction by CO[J]. J Phys Chem C, 2017, 123(23): 12757-12770. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=0c85386cb7ae05a7115af1236431c950 [7] LI K W, CHEN L H, WHITE S J, HAN K, LV B, BAO K J, WU X C, GAO X, AZZI M, CEN K F. Effect of nitrogen oxides (NO and NO2) and toluene on SO2, photooxidation, nucleation and growth: A smog chamber study[J]. Atmos Res, 2017, 192: 38-47. doi: 10.1016/j.atmosres.2017.03.017 [8] LU P, LI H, LIU H Y, CHEN Y F. Influence of tungsten on the NH3-SCR activity of Mn WOx/TiO2 catalysts[J]. Rsc Adv, 2017, 7(32): 19771-19779. doi: 10.1039/C7RA00427C [9] JIANG Y, XING Z M, WANG X C, HUANG S B, WANG X W, LIU Q Y. MoO3 modified CeO2/TiO2 catalyst prepared by a single step sol-gel method for selective catalytic reduction of NO with NH3[J]. J Ind Eng Chem, 2015, 29(1): 43-47. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=deb3b6ae8c0c2fcd192b47bb401604cc [10] MA L, SEO C Y, NAHATA M, CHEN X Y, LI J H, SCHWANK J W. Shape dependence and sulfate promotion of CeO2 for selective catalytic reduction of NOx with NH3[J]. Appl Catal B: Environ, 2018, 232(1): 246-259. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=07323cbbb93c6a0dd7cccc469be58026 [11] XU L W, WANG C Z, CHANG H Z, WU Q R. New insight into SO2 poisoning and regeneration of CeO2-WO3/TiO2 and V2O5-WO3/TiO2 catalysts for low-temperature NH3-SCR[J]. Environ Sci Technol, 2018, 52(12): 7064-7071. doi: 10.1021/acs.est.8b01990 [12] CHEN L, LI J H, GE M F. The poisoning effect of alkali metals doping over nano V2O5-WO3/TiO2 catalysts on selective catalytic reduction of NOx by NH3[J]. Chem Eng J, 2011, 170(2/3): 531-537. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=399fd63147e1906cfaa41d3df0ae8a45 [13] WANG H, QU Z P, DONG S C, TANG C. Mechanistic investigation into the effect of sulfuration on the FeW catalysts for the selective catalytic reduction of NOx with NH3[J]. ACS Appl Mater Inter, 2017, 9(8): 7017-7028. doi: 10.1021/acsami.6b14031 [14] PI Z P, SHEN B X, LIU J C, LIU Y F, ZHAO J G. Reduction of NOx in fluid catalytic cracking flue gas over Mg-Al spinel modified with transition metal oxides[J]. Pet Sci Technol, 2016, 34(24): 1958-1963. doi: 10.1080/10916466.2016.1236275 [15] JIANG R Y, SHAN H H, ZHANG J L, YANG C H, LI C Y. Synthesis, Characterization and evaluation of sulfur transfer catalysts for FCC flue gas[J]. China Pet Process Petrochem Technol, 2014, 16(2): 59-64. [16] KONG L P, MIAO J, LI M H, TAN G X, JIN G Z. CuMnCeLa-O/γ-Al2O3 catalytic combustion denitration performance research[J]. J Mol Catal, 2018, 32(4): 295-304. [17] MU J C, LI X Y, SUN W B, FAN S Y. Enhancement of low-temperature catalytic activity over highly dispersed Fe-Mn/Ti catalyst for selective catalytic reduction of NOx with NH3[J]. Ind Eng Chem Res, 2018, 57(31): 10159-10169. doi: 10.1021/acs.iecr.8b01335 [18] XU H D, ZHANG Q L, QIU C T, LIN T, GONG M C, CHEN Y Q. Tungsten modified MnOx-CeO2/ZrO2 monolith catalysts for selective catalytic reduction of NOx with ammonia[J]. Chem Eng Sci, 2012, 76: 120-128. doi: 10.1016/j.ces.2012.04.012 [19] TIAN G L, CUI S P, WANG Y L, WANG J F. Different precipitant preparation of nickel-doped Mn/TiO2 catalysts for low-temperature SCR of NO with NH3[J]. Mater Sci Forum, 2018, 913(1): 976-984. [20] ZHAO H J, FANG K G, DONG F, LIN M G, SUN Y H, TANG Z C. Textual properties of Cu-Mn mixed oxides and application for methyl formate synthesis from syngas[J]. Ind Eng Chem, 2017, 54(1): 117. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=d3c8ee27a10a61f86ccb7eb24d6b1bc7 [21] HE R X, WANG D D, ZHI K D, WANG B, ZHOU H C, JIANG H Q, LI N, LIU Q S. Cu-Mn catalysts modified by rare earth lanthanum for low temperature water-gas shift reaction[J]. J Rare Earth, 2016, 34(10): 994-1003. doi: 10.1016/S1002-0721(16)60126-6 [22] QI G, YANG R T. Characterization and FTIR studies of MnOx-CeO2 catalyst for low-temperature selective catalytic reduction of NO with NH3[J]. J Phys Chem B, 2004, 108(40): 15738-15747. doi: 10.1021/jp048431h [23] KANG M, PARK E D, KIM J M, YIE J E. Manganese oxide catalysts for NOx reduction with NH3 at low temperatures[J]. Appl Catal A: Gen, 2007, 327(2): 261-269. doi: 10.1016/j.apcata.2007.05.024 [24] XIA F T, SONG Z X, LIU X, LIU X, YANG Y H, ZHANG Q L, PENG J H. Improved catalytic activity and N2 selectivity of Fe-Mn-Ox catalyst for selective catalytic reduction of NO by NH3 at low temperature[J]. Res Chem Intermed, 2018, 44(1): 1-15. [25] LU H F, KONG X X, HUANG H F, ZHOU Y, CHEN Y F. Cu-Mn-Ce ternary mixed-oxide catalysts for catalytic combustion of toluene[J]. J Environ Sci, 2015, 32(6): 102-107. http://d.old.wanfangdata.com.cn/Periodical/jes-e201506012 [26] ZHANG L, CHEN T J, ZENG S H, SU H Q. Effect of doping elements on oxygen vacancies and lattice oxygen in CeO2-CuO catalysts[J]. J Environ Chem Eng, 2016, 4(3): 2785-2794. doi: 10.1016/j.jece.2016.05.023 -





 下载:
下载: