Synthesis of ZSM-5 zeolites with different silica/alumina ratios and their performance in the removal of aniline and pyridine from model fuel through adsorption
-
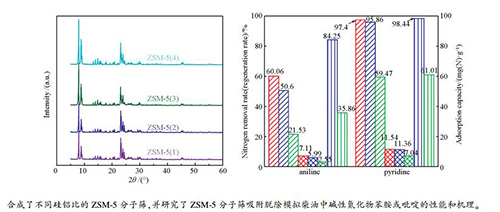
摘要: 合成了一系列不同硅铝比的ZSM-5分子筛,采用XRD、FT-IR、ICP、SEM、NH3-TPD和N2吸附-脱附等方法对其进行了表征,研究了不同硅铝比ZSM-5分子筛对模拟柴油中苯胺和吡啶的吸附脱除性能。结果表明,所合成的ZSM-5分子筛均具有典型MFI结构;与合成原料混合物中的硅铝比相比,实际硅铝比稍有降低。ZSM-5分子筛的酸量随硅铝比的增加而降低,硅铝比较小的ZSM-5(1)和ZSM-5(2)的吸附脱除苯胺或吡啶的效果明显优于其他样品,并且所有样品吸附脱除吡啶的效果均优于苯胺。ZSM-5(2)上苯胺和吡啶的吸附等温线符合Langmuir-Freundlich混合模型。Abstract: A series of ZSM-5 zeolites with different silica/alumina ratios were synthesized and characterized by means of XRD, FT-IR, ICP, SEM, NH3-TPD and N2 sorption; their performance in the removal of aniline and pyridine from a model fuel through adsorption was then investigated. The results indicated that all the as-synthesized ZSM-5 zeolites have the MFI structure, although the actual silica/alumina ratio in the as-synthesized ZSM-5 zeolites is somewhat lower than that in the corresponding synthesis mixture. As expected, the acid amount of ZSM-5 zeolites decreases with the increase of the silica/alumina ratio. The adsorption denitrogenation performance of ZSM-5(1) and ZSM-5(2) with relatively lower silica/alumina ratios is superior to that of other two zeolite samples; meanwhile, the removal efficiency for pyridine over all ZSM-5 samples is higher than that for aniline. Moreover, adsorption isotherms of aniline and pyridine over ZSM-5(2) accord with the Langmuir-Freundlich adsorption model.
-
Key words:
- ZSM-5 /
- silica-alumina ratio /
- aniline /
- pyridine /
- model fuel /
- adsorption denitrogenation
-
图 7 ZSM-5(2)对模拟柴油中苯胺的吸附等温线及其各等温吸附模型的拟合曲线
Figure 7 Adsorption isotherms of aniline over ZSM-5(2) (a) and those fitted with Langmuir, Freundlich and Langmuir-Freundlich adsorption models at 303 K (b), 323 K (c) and 343 K (d)
(adsorption conditions: 15 mL model fuel; 1.5 g adsorbent; 303, 323 or 343 K; 0.5 h)
图 8 ZSM-5(2)对模拟柴油中吡啶的吸附等温线及其各等温吸附模型的拟合曲线
Figure 8 Adsorption isotherms of pyridine over ZSM-5(2) (a) and those fitted with Langmuir, Freundlich and Langmuir-Freundlich adsorption models at 303 K (b), 323 K (c) and 343 K (d)
(adsorption conditions: 15 mL model fuel; 1.5 g adsorbent; 303, 323 or 343 K; 0.5 h)
表 1 ZSM-5样品的ICP测试结果
Table 1 ICP results of the ZSM-5 samples
Sample Content w/% Silica/alumina ratio in synthesis system Silica/alumina ratio in ZSM-5 SiO2 Al2O3 ZSM-5(1) 92.28 7.47 25 21 ZSM-5(2) 96.26 3.88 50 42 ZSM-5(3) 97.36 2.43 75 68 ZSM-5(4) 98.75 0.98 200 172 表 2 ZSM-5样品的孔结构参数
Table 2 Textural properties of the ZSM-5 samples
Sample BET surface area A/(m2·g-1) Total pore volume v/(cm3·g-1) Average pore diameter d/nm ZSM-5(1) 463 0.224 1.965 ZSM-5(2) 448 0.218 1.946 ZSM-5(3) 377 0.276 2.929 ZSM-5(4) 460 0.237 2.061 表 3 Langmuir,Freundlich和Langmuir-Freundlich等温吸附模型拟合的相关参数
Table 3 Regression coefficients for the adsorption of aniline and pyridine on ZSM-5(2) with various adsorption models
Adsorbate Temperature T/K Langmuir Freundlich Langmuir-Freundlich qm KL×105 R2 n KF R2 qm Ka×105 n R2 Aniline 303 140.7 8.966 0.986 2.172 0.893 0.941 122.9 11.95 1.251 0.989 323 189.6 5.647 0.973 1.830 0.425 0.975 212.9 1.734 0.744 0.976 343 153.0 8.338 0.971 2.116 0.838 0.949 156.8 7.876 0.971 0.969 Pyridine 303 276.2 4.614 0.991 1.443 0.142 0.976 184.8 9.589 1.345 0.995 323 284.4 4.329 0.992 1.580 0.241 0.973 223.7 6.964 1.229 0.995 343 338.9 3.661 0.994 1.498 0.186 0.977 246.0 6.753 1.292 0.998 -
[1] LAREDO G C, LEYVA S, ALVAREZ R, TERESA MARES M, CASTILLO J, LUIS CANO J. Nitrogen compounds characterization in atmospheric gas oil and light cycle oil from a blend of Mexican crudes[J]. Fuel, 2002, 81(10):1341-1350. doi: 10.1016/S0016-2361(02)00047-9 [2] CHENG X, ZHAO T, FU X, HU Z. Identification of nitrogen compounds in RFCC diesel oil by mass spectrometry[J]. Fuel Process Technol, 2004, 85(13):1463-1472. doi: 10.1016/j.fuproc.2003.10.004 [3] GARCÍA-GUTIÉRREZ J L, LAREDO G C, FUENTES G A, GARCÍA-GUTIÉRREZ P, JIMÉNEZ-CRUZ F. Effect of nitrogen compounds in the hydrodesulfurization of straight-run gas oil using a CoMoP/g-Al2O3 catalyst[J]. Fuel, 2014, 138:98-103. doi: 10.1016/j.fuel.2014.08.008 [4] POLO P. The suppression of a basic nitrogen compound influence on hydrodesulfurization activity of dibenzothiophene in treated diesel over Al2O3 supported CoMo catalysits by ZrO2 as a secondary support[J]. Catal Commun, 2015, 62(5):89-94. https://www.sciencedirect.com/science/article/pii/S1566736715000217 [5] PRADO G H C, YUAN R, KLERK A D. Nitrogen removal from oil:A review[J]. Energy Fuels, 2017, 31:14-36. doi: 10.1021/acs.energyfuels.6b02779 [6] 刘伟, 焦化柴油氧化脱氮工艺研究[D].北京: 中国石油大学, 2011. http://cdmd.cnki.com.cn/Article/CDMD-10425-1011287221.htmLIU Wei. Process of oxidative denitrification of coking diesel[D]. Beijing: China University of Petroleum, 2011. http://cdmd.cnki.com.cn/Article/CDMD-10425-1011287221.htm [7] 王云芳, 刘伟, 袁倩, 李青松.焦化柴油氧化萃取脱氮技术研究[J].应用化工, 2011, 40(8):1430-1436. doi: 10.3969/j.issn.1671-3206.2011.08.037WANG Yun-fang, LIU Wei, YUAN Qian, LI Qing-song. Technology of oxidative denitrification combined with extraction for coking diesel[J]. Appl Chem Ind, 2011, 40(8):1430-1436. doi: 10.3969/j.issn.1671-3206.2011.08.037 [8] SCHMITT C C, CHIARO S S X, TAKESHITA E V, YAMAMOTO C I. Regeneration of activated carbon from babassu coconut refuse applied as a complementary treatment to conventional refinery hydrotreatment of diesel fuel[J]. J Clean Prod, 2016, 140:1465-1469. https://www.sciencedirect.com/science/article/pii/S0959652616315876 [9] WEN J, LIN H, HAN X, ZHENG Y, CHU W. Physicochemical studies of adsorptive denitrogenation by oxidized activated carbons[J]. Ind Eng Chem Res, 2017, 56(17):5033-5041. doi: 10.1021/acs.iecr.6b05015 [10] TAN P, XIE X Y, LIU X Q, PAN T, GU C, CHEN P F, ZHOU J Y, PAN Y C, SUN L B. Fabrication of magnetically responsive HKUST-1/Fe3O4 composites by dry gel conversion for deep desulfurization and denitrogenation[J]. J Hazard Mater, 2017, 321:344-352. doi: 10.1016/j.jhazmat.2016.09.026 [11] LAREDO G C, VEGA-MERINO P M, MONTOYA-DE FUENTE J A, MORA-VALLEJO R J, MENESES-RUIZ E, CASTILLO J J, ZAPATO-RENDÓN B. Comparison of the metal-organic framework MIL 101(Cr) versus four commercial adsorbents for nitrogen compounds removal in diesel feedstocks[J]. Fuel, 2016, 180:284-291. doi: 10.1016/j.fuel.2016.04.038 [12] MAMBRINI R V, SALDANHA A L M, ARDISSON J D, ARAUJO M H, MOURA F C C. Adsorption of sulfur and nitrogen compounds on hydrophobic bentonite[J]. Appl Clay Sci, 2013, 84(10):286-293. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=JJ0231779223 [13] BAIA L V, SOUZAA W C, DE SOUZA R J F, VELOSO C D O, CHIARO S S X, FIGUEIREDO C M A G. Removal of sulfur and nitrogen compounds from diesel oil by adsorption using clays as adsorbents[J]. Energy Fuels, 2017, 31(11):11731-11742. doi: 10.1021/acs.energyfuels.7b01928 [14] MUSHRUSH G W, QUINTANA M A, BAUSERMAN J W, WILLAUER H D. Post-refining removal of organic nitrogen compounds from diesel fuels to improve environmental quality[J]. J Environ Sci Health A, 2011, 46(2):176-180. doi: 10.1080/10934529.2011.532433 [15] 李红跃, 王雷, 张曼, 赵德智, 刘宝玉, 王立新.新型改性硅胶对碱氮的吸附行为[J].应用化工, 2016, 45(6):1027-1029. http://d.old.wanfangdata.com.cn/Periodical/sxhg201606007LI Hong-yue, WANG Lei, ZHANG Man, ZHAO De-zhi, LIU Bao-yu, WANG Li-xin. Adsorption behavior of new modified silica gel for nitrogen base[J]. Appl Chem Ind, 2016, 45(6):1027-1029. http://d.old.wanfangdata.com.cn/Periodical/sxhg201606007 [16] 洪新, 李云赫, 袁加成, 赵永华, 唐克.变色硅胶吸附脱除模拟柴油中各种碱性氮化物[J].燃料化学学报, 2018, 46(3):298-304. doi: 10.3969/j.issn.0253-2409.2018.03.006HONG Xin, LI Yun-he, YUAN Jia-cheng, ZHAO Yong-hua, TANG Ke. Various basic nitrogen compounds removal from model diesel by adsorption with allochroic silica gel[J]. J Fuel Chem Technol, 2018, 46(3):298-304. doi: 10.3969/j.issn.0253-2409.2018.03.006 [17] KIM J H, MA X, ZHOU A, SONG C. Ultra-deep desulfurization and denitrogenation of diesel fuel by selective adsorption over three different adsorbents:a study on adsorptive selectivity and mechanism[J]. Catal Today, 2006, 111(1):74-83. doi: 10.1016-j.cattod.2005.10.017/ [18] ALMARRI M, MA X, SONG C. Selective adsorption for removal of nitrogen compounds from liquid hydrocarbon streams over carbon and alumina-based adsorbents[J]. Ind Eng Chem Res, 2009, 48(48):951-960. http://cn.bing.com/academic/profile?id=606ebc523e45a87345cdd2fbd87eaf46&encoded=0&v=paper_preview&mkt=zh-cn [19] XIE L L, FAVRE-REGUILLON A, WANG X X, FU X, LEMAIRE M. Selective adsorption of neutral nitrogen compounds from fuel using ion-exchange resins[J]. J Chem Eng Data, 2010, 55(11):4849-4853. doi: 10.1021/je100446p [20] CHITANDA J M, MISRA P, ABEDI A, DALAI A K, ADJAYE J D. Synthesis and characterization of functionalized poly(glycidyl methacrylate)-based particles for the selective removal of nitrogen compounds from light gas oil:Effect of linker length[J]. Energy Fuels, 2015, 29(3):1881-1891. doi: 10.1021/ef502210z [21] MISRA P, CHITANDA J M, DALAI A J, ADJAYE J D. Selective removal of nitrogen compounds from gas oil using functionalized polymeric adsorbents:Efficient approach towards improving denitrogenation of petroleum feedstock[J]. Chem Eng J, 2016, 295:109-118. doi: 10.1016/j.cej.2016.03.024 [22] 徐如人, 庞文琴, 霍启升.分子筛与多孔材料化学[M].第二版, 北京:科学出版社, 2015.XU Ru-ren, PANG Wen-qin, HUO Qi-sheng. Chemistry-Zeolites and Porous Materials[M]. Second edition. Beijing:Science Press, 2015. [23] 徐晓宇, 孙悦, 沈健, 翟玉龙. HY和USY分子筛对模拟油品中碱性氮化物的吸附行为[J].化工进展, 2014, 33(4):1035-1040. http://d.old.wanfangdata.com.cn/Periodical/hgjz201404042XU Xiao-yu, SUN Yue, SHEN Jian, ZHAI Yu-long. Adsorption behavior of basic nitrides in model oil on HY and USY molecular sieves[J]. Chem Ind Eng Prog, 2014, 33(4):1035-1040. http://d.old.wanfangdata.com.cn/Periodical/hgjz201404042 [24] 洪新, 唐克. NaY分子筛的改性及吸附脱氮性能[J].燃料化学学报, 2015, 43(2):214-220. doi: 10.3969/j.issn.0253-2409.2015.02.012HONG Xin, TANG Ke. Modification and adsorptive denitrification of NaY molecular sieve[J]. J Fuel Chem Technol, 2015, 43(2):214-220. doi: 10.3969/j.issn.0253-2409.2015.02.012 [25] SONG H, YOU J A, LI B, CHEN C, HUANG J, ZHANG J. Synthsis, characterization and adsorptive denitrogenation performance of bimodal mesoporous Ti-HMS/KIL-2 composite:A comparative study on synthetic methodology[J]. Chem Eng J, 2017, 327:406-417. doi: 10.1016/j.cej.2017.06.055 [26] SHAHRIAR S A, LIN H, ZHENG Y. Adsorptive denitrogenation and desulfurization of diesel fractions by mesoporous SBA15-supported nickel(Ⅱ) phosphide synthesized through a novel approach of urea matrix combustion[J]. Ind Eng Chem Res, 2012, 51(44):14503-14510. doi: 10.1021/ie3015044 [27] 洪新, 李云赫, 赵永华, 唐克.介孔材料Co-MCM-41的合成及其吸附脱除各种碱性氮化物[J].燃料化学学报, 2018, 46(2):243-250. doi: 10.3969/j.issn.0253-2409.2018.02.015HONG Xin, LI Yun-he, ZHAO Yong-hua, TANG Ke. Preparation of mesoporous Co-MCM-41 and its performance in adsorption removal of various basic nitrogen compounds[J]. J Fuel Chem Technol, 2018, 46(2):243-250. doi: 10.3969/j.issn.0253-2409.2018.02.015 [28] TANG K, HONG X. Preparation and characterization of Co-MCM-41 and its adsorption removing basic nitrogen compounds from FCC diesel oil[J]. Energy Fuels, 2016, 30(6):4619-4624. doi: 10.1021/acs.energyfuels.6b00427 [29] XUE T, WANG Y M, HE M Y. Synthsis of ultra-high-silica ZSM-5 zeolites with tunable crystal sizes[J]. Solid State Sci, 2012, 14(4):409-418. doi: 10.1016/j.solidstatesciences.2012.01.023 [30] 李兆飞, 郭成玉, 王骞, 刘其武, 邢昕, 胡云峰.不同硅铝比ZSM-5分子筛的合成及其在丁烯催化裂解中的应用[J].石油化工, 2016, 45(2):163-168. doi: 10.3969/j.issn.1000-8144.2016.02.007LI Zhao-fei, GUO Cheng-yu, WANG Qian, LIU Qi-wu, XING Xin, HU Yun-feng. Synthesis of ZSM-5 zeolites with different silica-aluminaratio and their application in catalytic cracking of 1-butene[J]. Petrochem Technol, 2016, 45(2):163-168. doi: 10.3969/j.issn.1000-8144.2016.02.007 [31] LI J, LIU S Y, ZHANG H, LÜ E J, REN P J, REN J. Synthesis and characterization of an unusual snowflake-shaped ZSM-5 zeolite with high catalytic performance in the methanol to olefin reaction[J]. Chin J Catal, 2016, 37(2):308-305. doi: 10.1016/S1872-2067(15)60979-2 [32] ZHANG S G, HIGASHINMOTO S, YAMASHITA H, ANPO M. Characterization of vanadium oxide/ZSM-5 zeolite catalysts prepared by the solid-state reaction and their photocatalytic reactivity:In situ photoluminescence, XAFS, ESR, FT-IR, and UV vis investigations[J]. J Phys Chem B, 1998, 102(29):5590-5594. doi: 10.1021/jp981230r [33] WIESSCHE C I D, MARTENS R, ZADRAZIL F. XAFS, IR and UV-vis study of the Cu-I environment in Cu-I-ZSM-5[J]. J Phys Chem B, 2014, 101(3):870-894. https://www.researchgate.net/publication/231656696_XAFS_IR_and_UV-vis_study_of_the_Cu-I_environment_in_Cu-I-ZSM-5 [34] 罗晓鸣, 王晓春, 张晓光, 许叔平, 陈旭.不同硅铝比ZSM-5分子筛性能的比较[J].石油学报(石油加工), 1986, 2(4):49-56. http://cdmd.cnki.com.cn/Article/CDMD-10270-1012454433.htmLUO Xiao-ming, WANG Xiao-chun, ZHANG Xiao-guang, XU Shu-ping, CHEN Xu. Comparison of the properties of ZSM-5 zeolites of different SiO/Al2O3 ratio[J]. Acta Pet Sin (Pet Process Sect), 1986, 2(4):49-56. http://cdmd.cnki.com.cn/Article/CDMD-10270-1012454433.htm [35] 姜玄珍, 郑雷.用原位红外光谱研究低硅铝比ZSM-5沸石中的新型B酸[J].分子催化, 1996, 10(3):183-186. http://www.cnki.com.cn/Article/CJFDTotal-FZCH603.004.htmJIANG Xuan-zhen, ZHENG Lei. Study on a novel typr of brönsted acid sites in ZSM-5 zeolite with low silicon-aluminum ratio by in-situ FT-IR technique[J]. J Mol Catal (China), 1996, 10(3):183-186. http://www.cnki.com.cn/Article/CJFDTotal-FZCH603.004.htm [36] 卢仁杰, 张新艳, 郝郑平.不同硅铝比Fe-ZSM-5催化剂对氧化亚氮催化分解性能的研究[J].环境科学, 2014, 35(1):371-379. http://d.old.wanfangdata.com.cn/Periodical/hjkx201401053LU Ren-jie, ZHANG Xin-yan, HAO Zheng-ping. Fe-ZSM-5 catalysts with different silica-alumina ratio for N2O catalytic decomposition[J]. Environ Sci, 2014, 35(1):371-379. http://d.old.wanfangdata.com.cn/Periodical/hjkx201401053 [37] MOURA R A, SEOLATTO A A, DE OLIVEIRA M, FREITAS F F. The adsorption study of royal blue tiafix and black tiassolan dyes using bone char as adsorbent[J]. Adsorpt Sci Technol, 2018, (3/4):1178-1198. https://www.researchgate.net/publication/323227162_The_adsorption_study_of_Royal_Blue_Tiafix_and_Black_Tiassolan_dyes_using_bone_char_as_adsorbent [38] SOARES J C, SOARES A C, PEREIRA P A R, RODRIGUES V D C, SHIMIZU F M, MELENDEZ M E, NETO C S, CARVALHO A L, LEITE F L, MACHADO S A S, OLIVEIRA JR O N. Adsorption according to the Langmuir-Freundlich model is the detection mechanism of the antigen p53 for early diagnosis of cancer[J]. Phys Chem Cheml Phys, 2016, 18(12):8412-8418. doi: 10.1039/C5CP07121F -





 下载:
下载: