Removal of Hg0 from simulated coal-fired flue gas by using activated spent FCC catalysts
-
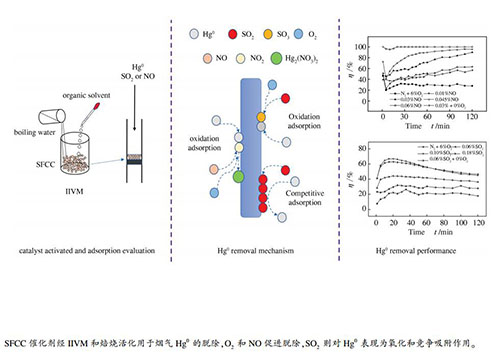
摘要: 采用有机溶剂内部瞬间沸腾法活化废流体催化裂化(SFCC)催化剂,通过固定床反应器考察了吸附温度、烟气成分及活化条件等多种变量对SFCC催化剂脱汞性能的影响。结果表明,SFCC催化剂经甲醇和乙醇活化后脱汞(Hg0)效果良好,焙烧温度也会影响催化剂活性。烟气中O2的存在有利于汞的脱除。含O2气氛下,NO在FCC-E催化剂表面形成含N活性位点,促进汞的脱除。SO2因浓度差异对Hg0的脱除表现出吸附催化和竞争吸附。在6% O2、12% CO2、0.06% NO烟气组分,120 ℃吸附温度,120 ℃活化温度,乙醇活化条件下,SFCC催化剂的脱汞效率接近100%。采用X射线荧光光谱(XRF)、扫描电镜(SEM)、X射线衍射(XRD)、热重(TG)和X射线光电子能谱(XPS)表征活化和未活化SFCC催化剂,探究与其脱汞性能关联。Abstract: The spent fluid catalytic cracking (SFCC) catalysts were activated by an "internal instant vaporization (ⅡV)" method and used in the removal of Hg0 from a simulated flue gas in a fixed bed reactor; the effect of various operation parameters such as the SFCC activation conditions, adsorption temperature, and flue gas components on the Hg0 removal efficiency was investigated. The results indicate that the SFCC catalyst activated with methanol or ethanol performs adequately in terms of Hg0 removal, whilst the calcination temperature also has a great influence on the activation of the SFCC catalyst. O2 in the flue gas favors the Hg0 removal, whilst NO facilitates the oxidation of mercury and displays a positive effect on the mercury removal in the presence of O2, accompanying with the formation of N-containing active species on the activated SFCC catalyst surface. SO2 in the flue gas, depending on its concentration, may exert the effect of catalytic adsorption or competitive adsorption on the Hg0 removal. Approximately 100% Hg0 can be removed in the stream of 6% O2, 12% CO2 and 0.06% NO at 120 ℃ by using the activated SFCC catalyst with ethanol as an organic solvent and calcined at 120 ℃, suggesting that the spent FCC catalysts after activation can be a potential adsorbent for the removal of Hg0 from the coal-fired flue gas.
-
Key words:
- spent FCC catalyst /
- activation /
- gaseous elemental mercury /
- coal-fired flue gas
1) 本文的英文电子版由Elsevier出版社在ScienceDirect上出版(http://www.sciencedirect.com/science/journal/18725813). -
Figure 6 Effect of the calcination temperature on the Hg0 removal efficiency of activated SFCC catalysts; the operation conditions of set I specified in Table 1 are used
Figure 7 Effect of the activation organic solvent on the Hg0 removal efficiency of the activated SFCC catalysts; the operation conditions of Set II specified in Table 1 are used
Figure 8 Effect of different adsorption temperature on the Hg0 removal efficiency of FCC-E; the operation conditions of Set III specified in Table 1 are used
Figure 9 Effect of O2 concentration in the flue gas on the Hg0 removal efficiency of FCC-E; the operation conditions of Set IV specified in Table 1 are used
Figure 10 Effect of SO2 concentration in the flue gas on the Hg0 removal efficiency of FCC-E; the operation conditions of Set V specified in Table 1 are used
Figure 11 Effect of NO concentration in the flue gas on the Hg0 removal efficiency of FCC-E; the operation conditions of Set VI specified in Table 1 are used
Table 1 Experimental conditions for the Hg0 adsorption tests
Set Adsorbent Activation conditions Flue gas composition Adsorption temp t/℃ calc. temp /℃ solvent I SFCC, activated SFCC 120-600 - 6% O2, 12% CO2, N2 120 II activated SFCC 120 methanol, ethanol, acetone, petroleum ether 6% O2, 12% CO2, N2 120 III activated SFCC 120 ethanol 6% O2, 12% CO2, N2 80-300 IV activated SFCC 120 ethanol 0-10%O2, 12% CO2, N2 120 V activated SFCC 120 ethanol 6% O2, 12% CO2, 0.06%-0.1% SO2, N2 120 VI activated SFCC 120 ethanol 6% O2, 12% CO2, 0.01%-0.06% NO, N2 120 Table 2 Textural properties of the activated and non-activated SFCC catalysts
Adsorbent Surface area A/(m2·g-1) Pore volume v/(cm3·g-1) Pore diameter d/nm SFCC 74.9 0.09 3.181 FCC-120 78.5 0.11 3.382 FCC-360 90.4 0.11 3.795 FCC-600 101.2 0.11 4.862 FCC-M 87.3 0.10 3.804 FCC-E 83.7 0.10 3.396 FCC-A 77.9 0.09 3.400 FCC-P 76.5 0.09 3.389 Table 3 Chemical composition of the SFCC catalysts determined by XRF
Component Percentage Component Percentage Component Percentage Al2O3 46.397 Cs2O 1.115 V2O5 0.169 SiO2 43.147 Na2O 0.782 CeO2 0.166 CaO 2.012 P2O5 0.714 Sb2O3 0.121 SO3 1.546 TiO2 0.302 ZnO 0.093 NiO 1.416 MgO 0.254 La2O3 0.089 Fe2O3 1.330 K2O 0.177 else 0.163 -
[1] CHEN Y, GUO X, FAN W. Development and evaluation of magnetic iron-carbon sorbents for mercury removal in coal combustion flue gas[J]. J Energy Inst, 2020, 93(4): 1615-1623. [2] UNEP, UNEP publishes 2018 global mercury assessment[R]. UN Environment Programme, Chemicals and Health Branch Geneva, Switzerland, 2019. [3] XIE X, AI H S, DENG Z G. Impacts of the scattered coal consumption on PM2.5 pollution in China[J]. J Clean Prod, 2020, 245: 118922. [4] LIU T, XUE L C, GUO X. Study of Hg0 removal characteristics on Fe2O3 with H2S[J]. Fuel, 2015, 160: 189-195. [5] GB/13223—2011, Emission standard of air pollutants for thermal power plants[S]. [6] WU S J, YAN P J, YU W S, CHENG K, WANG H, YANG W, ZHOU J, XI J H, QIU J H, ZHU S X, CHE L. Efficient removal of mercury from flue gases by regenerable cerium-doped functional activated carbon derived from resin made by in situ ion exchange method[J]. Fuel Process Technol, 2019, 196: 106167. [7] WU C L, CAO Y, DONG Z B, CHENG C M, LI H X, PAN W P. Evaluation of mercury speciation and removal through air pollution control devices of a 190 MW boiler[J]. J Environ Sci, 2010, 22: 277-282, [8] LEE SS, LEE J Y, KEENER T C. Bench-scale studies of in-duct mercury capture using cupric chloride-impregnated carbons[J]. Environ Sci Technol, 2009, 43: 2957-2962. [9] MEI Z J, SHEN ZM, ZHAO Q J, WANG W H, ZHANG Y J. Removal and recovery of gas-phase element mercury by metal oxide-loaded activated carbon[J]. J Hazard Mater, 2008, 152: 721-729. [10] YANG J P. ZHAO Y C, ZHANG J Y, ZHENG C G. Removal of elemental mercury from flflue gas by recyclable CuCl2 modified magnetospheres catalyst from fly ash. Part 1. analyst characterization and performance evaluation[J]. Fuel, 2016, 164: 419-428. [11] WANG Y J, DUAN Y F. Effect of manganese ions on the structure of Ca(OH)2 and mercury adsorption performance of Mnx+/Ca(OH)2 composites[J]. Energy Fuels, 2011, 25: 1553-1558. [12] LIU H, YANG J P, TIAN C, ZHAO Y C, ZHANG JY.Mercury removal from coal combustion flue gas by modified palygorskite adsorbents[J]. Appl Clay Sci, 2017. 147: 36-43. [13] YANG J P, ZHU W B, QU W Q, YANG Z Q, ZHAO J X, WANG J, ZHANG M G, LI H L. Selenium functionalized metal-organic framework MIL-101 for efficient and permanent sequestration of mercury[J]. Environ Sci Technol, 2019, 53: 2206-2268. [14] YANG Z Q, LI H L, QU W Q, ZHANG M G, FENG Y, ZHAO J X, YANG J P, SHI K M. Role of sulfur trioxide (SO3) in gas-phase elemental mercury immobilization by mineral sulfide[J]. Environ Sci Technol, 2019, 53: 3250-3257. [15] LI H L, ZHU W B, YANG J P, ZHANG M G, ZHAO J X, QU W Q. Sulfur abundant S/FeS2 for efficient removal of mercury from coal-fired power plants[J]. Fuel, 2018, 232: 476-484. [16] LIU H, ZHAO Y, ZHOU Y M, ZHANG J Y. Removal of gaseous elemental mercury by modified diatomite[J]. Sci Total Environ, 2019, 652: 651-659. [17] JOHNSON E B G, ARSHAD S E B. Arshad. Hydrothermally synthesized zeolites based on kaolinite: A review[J]. Appl Clay Sci, 2014: 97-98, 215-221. [18] ADITYA B, ANJANI S. Catalyst demand growth projected at 1.1% through 2040[J]. Huston. Stradv, 2019: 1-2 (2020-02-10) https: //stratasadvisors.com/insights/2019/030119-catalyst-market-outlook. [19] VUYYURU K, PANT KK, KRISHNANV V, NIGAM K D P. Recovery of nickel from spent industrial catalysts using chelating agents[J]. Ind Eng Chem Res, 2010, 49: 2014-2024. [20] HUANG Y Y, CHEN X P, DENG Y F, ZHOU D, WANG L L. A novel nickel catalyst derived from layered double hydroxides(LDHs) supported on fluid catalytic cracking catalyst residue(FC3R) for rosin hydrogenation[J]. Chem Eng J, 2015, 269: 434-443. [21] FERELLA F, LENOE S, INNOCENZI V, MICHELIS I D, TAGLIERI G, GALLUCCI K. Synthesis of zeolites from spent fluid catalytic cracking catalyst[J]. JClean Prod, 2019, 230: 910-926. [22] YUANL, QIU Z F, YUAN L, TARIQ M, LU Y Q, YANG J, LI Z, LYU S G. Adsorption and mechanistic study for phosphate removal by magnetic Fe3O4-doped spent FCC catalysts adorbent[J]. Chemosphere, 2019, 219: 183-190. [23] LIU H, CHANG L, LIU W J, XIONG Z, ZHAO Y C, ZHANG J Y, ZHANG J Y. Advances in mercury removal from coal-fired flue gas by mineral adsorbents[J]. Chem Eng J, 2020, 379: 122263. [24] RODRIGUEZE D, BERNAL SA, PROVIS J, GEHMAN J, MONZO J, PAYA J, BORRACHERO M V. Geopolymers based on spent catalyst residue from a fluid catalytic cracking (FCC) process[J]. Fuel, 2013, 109: 493-502. [25] DENG S, WANG H S, CHEN X P, WANG LL, LIANG J Z, ZHANG F. The preparation and application of a mercury adsorbent, CN, 201610524841.1[P]. 2016-07-05. [26] PAYA J, MONZO J, BORRACHERO M V, VELAZQUEZ S, BONILLA M. Determination of the pozzolanic activity of fluid catalytic cracking residue. Thermogravimetric analysis studies on FC3R-lime pastes[J]. Cement Concrete Res, 2003, 33: 1085-1091. [27] MA L J, HAN L N, CHEN S, HU J L, CHANG L Q, BAO W R, WANG J C. Rapid synthesis of magnetic zeolite materials from fly ash and iron-containing wastes using supercritical water for elemental mercury removal from flue gas[J]. Fuel Process Technol, 2019, 189: 39-48. [28] ZHANG Z, WU J, LI B, XU H B, LIU D J. Removal of elemental mercury from simulated flue gas by ZSM-5 modified with Mn-Fe mixed oxides[J]. Chem Eng J, 2019, 375: 121946. [29] LI H H, WANG Y, WANG S K, WANG X, HU JJ. Removal of elemental mercury in flue gas at lower temperatures over Mn-Ce based materials prepared by co-precipitation[J]. Fuel, 2017, 208: 576-586. [30] HE C, SHEN B X, LI FK. Effects of flue gas components on removal of elemental mercury over Ce-MnOx/Ti-PILCs[J]. J Hazard Mater, 2016, 304: 10-17. [31] LU G J, LU X Y, LIU P. Recovery of rare earth elements from spent fluid catalytic cracking catalyst using hydrogen peroxide as a reductant[J]. Miner Eng, 2020, 145: 106104. [32] SHI M T, LUO G Q, XU Y, ZOU R J, ZHU H L, HU J Y, LI X, YAO H. Using H2S plasma to modify activated carbon for elemental mercury removal[J]. Fuel, 2019, 254: 115549. [33] SUN R Z, ZHU H L, SHI M T, LUO G Q. Preparation of fly ash adsorbents utilizing non-thermal plasma to add S active sites for Hg0 removal from flue gas[J]. Fuel, 2020, 266: 116936. [34] DONG L, HUANG Y J, LIU L Q, LIU C Q, XU L G, ZHA J R, CHEN H, LIU H. Investigation of elemental mercury removal from coal-fired boiler flue gas over MIL101-Cr[J]. Energy Fuels, 2019, 33: 8864-8875. [35] SHEN F H, LIU J, WU D W, DONG Y C, LIU F, HUANG H. Design of O2/SO2 dual-doped porous carbon as superior sorbent for elemental mercury removal from flue gas[J]. J Hazard Mater, 2019, 366: 321-328. [36] TONG L, XU W Q, ZHOU X, LIU R H. Effects of multi-component flue gases on Hg0 removal over HNO3-modified activated carbon[J]. Energy Fuels, 2015, 29: 5231-5236. [37] LI H L, WU C Y, LI Y, LI L Q, ZHAO Y C, ZHANG J Y. Impact of SO2 on elemental mercury oxidation over CeO2-TiO2 catalyst[J]. ChemEng J, 2013, 219: 319-326. [38] ZHOU C S, SUN L S, ZHANG A C, MA C, WANG B, YU J, SU S, HU S, XIANG J. Elemental mercury (Hg0) removal from containing SO2/NO flue gas by magnetically separable Fe2.45Ti0. 55O4/H2O2, advanced oxidation processes[J]. Chem Eng J, 2015, 273: 381-389. [39] LIUR H, XU W Q, TONG L, ZHU T Y. Role of NO in Hg0 oxidation over a commercial selective catalytic reduction catalyst V2O5-WO3/TiO2[J]. J Environ Sci, 2015, 38: 126-132. [40] YANG Y J, MIAO S, LIU J, WANG Z, YU YN. Cost-effective manganese ore sorbent for elemental mercury removal from flue gas[J]. Environ Sci Technol, 2019, 53: 9957-9965. [41] LUO Z K, DUAN Y F, HUANG T F, LIU S, HUANG Y J, DONG L, REN S J, TAO J, GU X B. Emission and migration characteristics of mercury in a 0.3 MWth CFB Boiler with ammonium bromide-modified rice husk char injection into flue[J]. Energy Fuels, 2019, 33: 7578-7586. [42] RUMAYOR M, DIAZ-SOMOANO M, LOPEZ-ANTON M A, OCHOA-GONZALEZ R, MARTINEZ-TARAZONA M R. Temperature programmed desorption as a tool for the identification of mercury fate in wet-desulphurization systems[J]. Fuel, 2015, 148: 98-103. -





 下载:
下载: