Study on the performance of CF3SO3H modified sulfonic polymer-based catalyst in formaldehyde carbonylation reaction
-
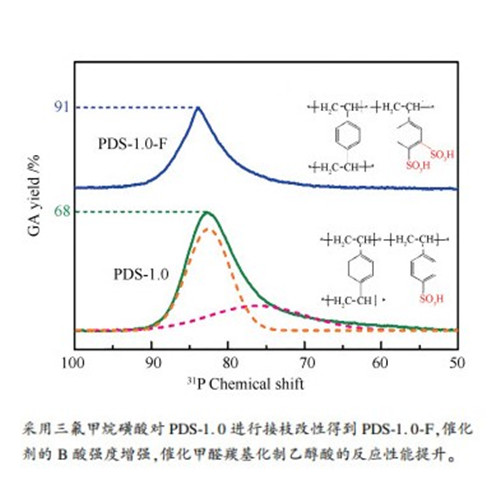
摘要: 采用水热合成、原位磺化法制备了固体磺酸型聚合物PDS-1.0催化剂,以三氟甲烷磺酸对其进行接枝改性得到PDS-1.0-F催化剂;采用N2吸附-脱附、TG、FT-IR、31P MAS NMR和XPS等技术对催化剂的物理和化学性质进行了表征,以甲醛羰基化制乙醇酸为探针反应对其催化性能进行了评价研究。结果表明,与PDS-1.0催化剂相比,氟磺酸改性后的PDS-1.0-F催化剂的比表面积、孔容积和酸量均降低,但是酸强度和热稳定性显著增加,由此对甲醛羰基化反应具有较好的催化性能,乙醇酸收率达到91.2%。Abstract: A sulfonic polymer-based catalyst (PDS-1.0) was synthesized by the hydrothermal and in situ sulfonation method, which is further modified by grafting with CF3SO3H, to get the PDS-1.0-F catalyst. The physical and chemical properties of the PDS-1.0 and PDS-1.0-F catalysts were characterized by N2 sorption, TG, FT-IR, 31P MAS NMR and XPS; the effect of CF3SO3H modification on the catalytic performance of PDS-1.0-F in the carbonylation of formaldehyde to glycolic acid was then investigated. The results show that after the CF3SO3H modification, the framework of PDS-1.0-F catalyst was grafted with strong electron withdrawing groups. In comparison with PDS-1.0, the CF3SO3H-modified PDS-1.0-F catalyst has a lower surface area, pore volume and concentration of acid sites, but much stronger acidity and higher thermal stability. As a result, the PDS-1.0-F catalyst exhibits excellent performance in the carbonylation of formaldehyde and over it the yield of glycolic acid reaches 91.2%.
-
表 1 催化剂的织构性质及表面酸性
Table 1 Textural and acidic parameters of various catalysts
Catalyst ABET/(m2·g-1) vp/(cm3·g-1) dp/nm S contenta /(mmol·g-1) Acid sitesb /(mmol·g-1) PDS-1.0 320 0.65 7.7 1.95 1.90 PDS-1.0-F 226 0.67 10.1 3.69 1.65 ABET: BET surface area; vp: BJH pore volume; dp: average pore diameter; a: measured by CHNS elemental analysis; b: measured by acid-base titration 表 2 催化剂的甲醛羰基化反应的评价
Table 2 Catalytic performance of PDS-1.0 and PDS-1.0-F in the carbonylation of formaldehyde
Catalyst Conversion /% Yield /% STY None 0 0 - PDS-1.0 99.9 68.8 36.9 PDS-1.0-F 99.9 91.2 46.0 CF3SO3H 99.8 94.8 10.9 reaction conditions: 120℃, 6.0MPa, reaction time = 12h, TOX:Catalyst:HAc (mass) = 1:0.4: 9, conversion: formaldehyde conversion, yield: glycolic acid yield, STY: space time yield -
[1] HAL J W V, LEDFORD J S, ZHANG X. Investigation of three types of catalysts for the hydration of ethylene oxide (EO) to monoethylene glycol (MEG)[J]. Catal Today, 2007, 123(1/4):310-315. https://www.sciencedirect.com/science/article/pii/S0920586107001149 [2] 杨爱民.乙二醇市场回顾及发展前景[J].聚酯工业, 2018, 31(3):5-7. doi: 10.3969/j.issn.1008-8261.2018.03.002YANG Ai-min. Ethylene glycol market review and development prospect[J]. Polyester Ind, 2018, 31(3):5-7). doi: 10.3969/j.issn.1008-8261.2018.03.002 [3] 宋河远, 靳荣华, 康美荣, 陈静.碳一化工路线制备乙二醇研究进展[J].催化学报, 2013, 34(6):1035-1050. http://www.cnki.com.cn/Article/CJFDTotal-CHUA201306005.htmSONG He-yuan, JIN Rong-hua, KANG Mei-rong, CHEN Jing. Progress in synthesis of ethylene glycol through C1 chemical industry routes[J]. Chin J Catal, 2013, 34(6):1035-1050. http://www.cnki.com.cn/Article/CJFDTotal-CHUA201306005.htm [4] 崔建方. C1化学路线合成乙二醇技术进展[J].煤化工, 2011, 39(4):9-12. doi: 10.3969/j.issn.1005-9598.2011.04.003CUI Jian-fang. Progress in synthesis of ethylene glycol through C1 chemical route[J]. Chem Ind, 2011, 39(4):9-12. doi: 10.3969/j.issn.1005-9598.2011.04.003 [5] 靳丽丽.乙二醇合成工艺研究进展[J].煤炭与化工, 2018, 41(2):35-37. http://www.cnki.com.cn/Article/CJFDTotal-HHGZ201802011.htmJIN Li-li. Progress on synthetic technologies of ethylene glycol[J]. Coal Chem Ind, 2018, 41(2):35-37. http://www.cnki.com.cn/Article/CJFDTotal-HHGZ201802011.htm [6] HUANG Y Y, MCCARTHY T J, SACHTLER W M H. Preparation and catalytic testing of mesoporous sulfated zirconium dioxide with partially tetragonal wall structure[J]. Appl Catal A:Gen, 1996, 148(1):135-154. doi: 10.1016/S0926-860X(96)00223-2 [7] OLAH G A. GKS PRAKASH, J. SOMMER. Superacids[J]. Science, 1979, 206:13-20. doi: 10.1126/science.206.4414.13 [8] GILLESPIE R J. Fluorosulfuric acid and related superacid media[J]. Acc Chem Res, 1968, 1(7):202-209. doi: 10.1021/ar50007a002 [9] HE D H, HUANG W G, LIU J Y, ZHU Q M. Condensation of formaldehyde and methyl formate to methyl glycolate and methyl methoxy acetate using heteropolyacids and their salts[J]. Catal Today, 1999, 51(1):127-134. doi: 10.1016/S0920-5861(99)00014-0 [10] HE D H, HUANG W G, LIU J Y, ZHU Q M. The activity of H4SiW12O40 for the coupling of formaldehyde and methyl formate to methyl glycolate and methyl methoxy acetate[J]. J Mol Catal A:Chem, 1999, 145(1/2):335-338. https://www.sciencedirect.com/science/article/pii/S1381116999001661 [11] LI T, SOUMA Y, XU Q. Carbonylation of formaldehyde catalyzed by p-toluenesulfonic acid[J]. Catal Today, 2006, 111(3/4):288-291. https://www.sciencedirect.com/science/article/abs/pii/S0920586105007935 [12] LIU S P, ZHU W L, SHI L, LIU H C, NI Y M, LIL N, ZHOU H, XU S T, HE Y L, LIU Z M. Activity enhancement of Nafion resin:Vapor-phase carbonylation of dimethoxymethane over Nafion-silica composite[J]. Appl Catal A:Gen, 2015, 497:153-159. doi: 10.1016/j.apcata.2015.03.010 [13] PIROZHKOV S D, STEPANYAN A S, MYSHENKOVA T N, ORDYAN M B, LAPIDUS A L. Carbonylation of olefins and alcohols at atmospheric pressure in the presence of acid catalysts with added Ag2O or Cu2O[J]. Russ Chem Bull, 1982, 31(9):1852-1858. doi: 10.1007/BF00952388 [14] LEE S, KIM J C, LEE J S, KIM Y G. Carbonylation of formaldehyde over ion exchange resin catalysts. 1. Batch reactor studies[J]. Ind Eng Chem Res, 1993, 32(2):253-259. doi: 10.1021/ie00014a002 [15] MELERO J A, VAN GRIEKEN R, MORALES G. Advances in the synthesis and catalytic applications of organosulfonic-functionalized mesostructured materials[J]. Chem Rev, 2006, 106(9):3790-3812. doi: 10.1021/cr050994h [16] LIU F J, ZHENG A M, NOSHADI I, XIAO F S. Design and synthesis of hydrophobic and stable mesoporous polymeric solid acid with ultra strong acid strength and excellent catalytic activities for biomass transformation[J]. Appl Catal B:Environ, 2013, 136:193-201. https://www.sciencedirect.com/science/article/pii/S0926337313000982 [17] YUAN D P, ZHAO N, WANG Y X, XUAN K, LI F, PU Y, WANG F, LI L, XIAO F K. Dehydration of sorbitol to isosorbide over hydrophobic polymer-based solid acid[J]. Appl Catal B:Environ, 2019, 240:182-192. doi: 10.1016/j.apcatb.2018.08.036 [18] BOTHE N, DOSCHER F, KLEIN J, WIDDECKE H. Thermal stability of sulphonated styrene-divinylbenzene resins[J]. Polym, 1979, 20(7):850-854. https://www.sciencedirect.com/science/article/abs/pii/0032386179901228 [19] WIDDECKE H. Polystyrene-supported acid catalysis[J]. Br Polym J, 1984, 16(4):188-192. doi: 10.1002/pi.4980160406 [20] LV H Y, ZHAO X L, LI Z D, WANG Z L, YANG X N. Fluorinated low band gap copolymer based on dithienosilole-benzothiadiazole for high-performance photovoltaic device[J]. Polym Chem, 2014, 5(21):6279-6286. doi: 10.1039/C4PY00758A [21] SUN Q, HU K W, LENG K Y, YI X F, AGULIA B, SUN Y Y, ZHENG A M, MENG X J, MA S Q, XIAO F S. A porous Brønsted superacid as an efficient and durable solid catalyst[J]. J Mate Chem A, 2018, 6(38):18712-18719. doi: 10.1039/C8TA06516K [22] LIU F J, KONG W P, Qi C Z, ZHU L F, XIAO F S. Design and synthesis of mesoporous polymer-based solid acid catalysts with excellent hydrophobicity and extraordinary catalytic activity[J]. ACS Catal, 2012, 2(4):565-572. doi: 10.1021/cs200613p [23] LIU F J, MENG X G, ZHANG Y L, REN L M, NAWAZ F, XIAO F S. Efficient and stable solid acid catalysts synthesized from sulfonation of swelling mesoporous polydivinylbenzenes[J]. J Catal, 2010, 271(1):52-58. https://www.sciencedirect.com/science/article/pii/S0021951710000369 [24] KOLBECK C, KILLIAN M, MAIER F, PAAPE N, WASSERSCHEID P, STEINRUCK H P. Surface characterization of functionalized imidazolium-based ionic liquids[J]. Langmuir, 2008, 24(17):9500-9507. doi: 10.1021/la801261h [25] YUAN D P, LI L, WANG Y X, WANG F, ZHAO N, XIAO F K. Solvent-free production of Isosorbide from sorbitol catalyzed by a polymeric solid acid[J]. ChemSusChem, 2019, 12(22):4986-4995. doi: 10.1002/cssc.201901922 [26] ZHANG X M, ZHAO Y P, YANG Q H. PS-SO3H@phenylenesilica with yolk-double-shell nanostructures as efficient and stable solid acid catalysts[J]. J Catal, 2014, 320:180-188. doi: 10.1016/j.jcat.2014.09.018 [27] ZHENG A M, HUANG S J, LIU S B, DENG F. Acid properties of solid acid catalysts characterized by solid-state 31P NMR of adsorbed phosphorous probe molecules.[J]. Phys Chem Chem Phys, 2011, 13(33):14889-14901. doi: 10.1039/c1cp20417c [28] TAGUSAGAWA C, TAKAGAKI A, IGUCHI A, TAKANABE K, KONDO J N, EBITANI K, HAYASHI S, TATSUMI T, DOMEN K. Highly active mesoporous Nb-W oxide solid-acid catalyst[J]. Angew Chem, 2010, 49(6):1128-1132. doi: 10.1002/anie.200904791 [29] SONG H Y, LI Z, CHEN J, XIA C G. Brönsted acidic ionic liquids as efficient and recyclable catalysts for the carbonylation of formaldehyde[J]. Catal Lett, 2012, 142(1):81-86. doi: 10.1007/s10562-011-0708-x -





 下载:
下载: