-
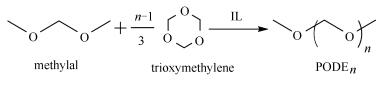
摘要: 聚甲氧基二甲醚(H3CO(CH2O)nCH3,PODEn或DMMn,n ≥ 2)具有独特的物理化学性质;作为一种柴油添加剂,可以有效提高油品燃烧效率并达到节能减排的目的。首先合成了一系列聚合度n为2、3、4和5单一组分的聚甲氧基二甲醚,采用NMR、FT-IR、Raman和DFT计算等手段对每个聚甲氧基二甲醚单体的化学结构进行表征,并对其在298.15-323.15K温度的密度和黏度进行了测试。结果表明,聚甲氧基二甲醚的密度和黏度随着温度的升高而逐渐降低,随着聚合度的增加而逐渐升高。同时,聚甲氧基二甲醚PODEn(n =2-5)的闪点和倾点以及溶解热和凝固热均随着聚合度的增加而提高。Abstract: Polyoxymethylene dimethyl ethers (H3CO(CH2O)nCH3, PODEn or DMMn, n ≥ 2) with unique physical and chemical properties are a potential additive for diesel fuels, which can effectively enhance the combustion efficiency and reduce the emission of pollutants. In this work, a series of pure PODEn components (n=2-5) were synthesized from methylal and trioxymethylene and obtained with high purity through collaborative separation; their structure and properties were characterized by NMR, FT-IR, Raman, and DFT calculation and a detailed assignment of the expressions in the spectrogram to the various groups was performed. The density and viscosity of PODEn were measured at 298.15-323.15 K. The results indicate that the density and viscosity of PODEn decrease gradually with the increase of temperature. Meanwhile, with the increase in the number of -CH2O-units (n) from 2 to 5, the density, viscosity, flash point, pour point, and the heat of fusion and solidification of PODEn are all increased. These results are valuable for the practical synthesis and application of PODEn.
-
Table 1 Comparison of the experimental and calculated vibration wave number and the assignment of various radicals in PODEn

Table 2 Density of PODEn under different temperatures

Table 3 Viscosity of PODEn under different temperatures

Table 4 Flash point and pour point of PODEn

Table 5 Thermal characteristics of PODEn

-
[1] UCHIDA T, KURITA Y, KUBO M. The dipole moments and the strucures of polyoxymethylene dimethyl ethers[J]. J Polym Sci, 1956, 19(92):365-372. doi: 10.1002/pol.1956.120199215 [2] ARVIDSON M, FAKLEY M E, SPENCER M S. Lithium Halide-Assisted formation of polyoxymethylene dimethyl ethers from dimethoxymethane and formaldehyde[J]. J Mol Catal, 1987, 41(3):391-393. doi: 10.1016/0304-5102(87)80118-9 [3] VIGIER F, COUTANCEAU C, LÉGERJ M, DUBOIS J L. Polyoxymethylene dimethyl ether (CH3O(CH2O)nCH3) oxidation on Pt and Pt/Ru supported catalysts[J]. J Power Sources, 2008, 175(1):82-90. doi: 10.1016/j.jpowsour.2007.09.053 [4] MASAHIRO W, HIROYUKI U, STEVE B, JEAN-LUC D. Fuel cells using an oxy-carbon fuel soluble in aqueous meduim: EP, 1993159A1[P]. 2008-11-19. [5] BURGER J, SIEGERT M, STRÖFER E, HASSE H. Poly(oxymethylene) dimethyl ethers as components of tailored diesel fuel:Properties, synthesis and purification concepts[J]. Fuel, 2010, 89(11):3315-3319. doi: 10.1016/j.fuel.2010.05.014 [6] ZHAO Q, WANG H, QIN ZHF, WU ZH W, WU J B, FAN W B, WANG J G. Synthesis of polyoxymethylene dimethyl ethers from methanol and trioxymethylene with molecular sieves as catalysts[J]. J Fuel Chem Technol, 2011, 39(12):918-923. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract17845.shtml [7] WU J B, WU Z W, WANG R Y, SHI R P, QIN Z F, ZHU H Q, DONG M, FAN W B, WANG J G. Recent research progresses in the catalytic synthesis of methyl formate, dimethoxymethane and polyoxymethylene dimethyl ethers from methano[J]. J Fuel Chem Technol, 2015, 43(7):816-828. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18658.shtml [8] GAO X C, YANG W M, LIU Z C, GAO H X. Catalytic performance of HZSM-5 molecular sieve for synthesis of polyoxymethylene dimethyl ethers[J]. Chin J Catal, 2012, 33(8):1389-1394. https://www.researchgate.net/publication/275884405_Catalytic_Performance_of_HZSM-5_Molecular_Sieve_for_Synthesis_of_Polyoxy-methylene_Dimethyl_Ethers [9] LI H J, SONG H L, ZHAO F, CHEN L W, XIA CH G. Chemical equilibrium controlled synthesis of polyoxymethylene dimethyl ethers over sulfated titania[J]. J Energy Chem, 2015, 24(2):239-244. doi: 10.1016/S2095-4956(15)60307-2 [10] REN Y, HUANG Z H, MIAO H Y, DI Y G, JIANG D M, ZENG K, LIU B, WANG X B. Combustion and emissions of a DI diesel engine fuelled with diesel-oxygenate blends[J]. Fuel, 2008, 87(12):2691-2697. doi: 10.1016/j.fuel.2008.02.017 [11] ZHAO Y P, XU Z, CHEN H, FU Y C, SHEN J Y. Mechanism of chain propagation for the synthesis of polyoxymethylene dimethyl ethers[J]. J Energy Chem, 2013, 22(6):833-836. doi: 10.1016/S2095-4956(14)60261-8 [12] BURGER J, STRÖFER E, HASSE H. Chemical equilibrium and reaction kinetics of the heterogeneously catalyzed formation of poly(oxymethylene) dimethyl ethers from methylal and trioxane[J]. Ind Eng Chem Res, 2012, 51(39):12751-12761. doi: 10.1021/ie301490q [13] CHEN J, SONG H Y, XIA C G, KANG M R, JIN R H. System and method for continuously producing polyoxymethylene dialkyl ethers: AU, 2012268915[P]. 2014-05-15. [14] CHEN J, SONG H Y, XIA C G, LI Z. Method for synthesizing polyoxymethylene dimethyl ethers catalyzed by an ionic liquid: US, 0288343[P]. 2011-11-24. [15] SCOTT A P, RADOM L. Harmonic vibrational frequencies:An evaluation of Hartree-Fock, Moller-Plesset, quadratic configuration interaction, density functional theory, and semiempirical scale factors[J]. J Phys Chem, 1996, 100(41):16502-16513. doi: 10.1021/jp960976r [16] PARLAK C. Theoretical and experimental vibrational spectroscopic study of 4-(1-pyrrolidinuyl) piperidine[J]. J Mol Struct, 2010, 966(1):1-7. http://www.sciencedirect.com/science/article/pii/S0022286009007558 [17] DIKMEN G, ALVER Ö. NMR, FT-IR, Raman and UV-Vis spectroscopic investigation and DFT study of 6-bromo-3-pyridinyl boronic acid[J]. J Mol Struct, 2015, 1099:625-632. doi: 10.1016/j.molstruc.2015.05.063 [18] ANDERSSON M P, UVDAL P. New scale factors for harmonic vibrational frequencies using the B3LYP density functional method with the triple-ξ basis set 6-311+G(d, p)[J]. J Phys Chem A, 2005, 109(12):2937-2941. doi: 10.1021/jp045733a [19] SONG D Y, CHEN J. Densities and viscosities for ionic liquids mixtures containing[eOHmin][BF4], [bmim][BF4] and[bpy][J]. J Chem Thermodyn, 2014, 77:137-143. doi: 10.1016/j.jct.2014.05.016 [20] MEHRDAD A, NIKNAM Z. Investigation on the interactions of poly(ethylene oxide) and ionic liquid 1-butyl-3-methyl-imidazolium bromide by viscosity and spectroscopy[J]. J Chem Eng Data, 2016, 61(5):1700-1709. doi: 10.1021/acs.jced.5b00428 [21] LINTON W H, GOODMAN H H. Physical properties of high molecular weight acetal resins[J]. J Appl Polym Sci, 1959, 1(2):179-184. doi: 10.1002/app.1959.070010208 [22] GUNBAS G, HAFEZI N, SHEPPARD W L, OLMSTEAD M M, STOYANOVA I V, THAM F S, MEYER M P, MASCAL M. Extreme oxatriquinanes and a record C-O bond length[J]. Nat Chem, 2012, 4(12):1018-1023. doi: 10.1038/nchem.1502 [23] ALLINGER N L, LⅡ J H, SCHAEFER H F. Molecular mechanics (MM4) studies on unusually long carbon-carbon bond distances in hydrocarbons[J]. J Chem Theory Comput, 2016, 12(6):2774-2778. doi: 10.1021/acs.jctc.5b00926 [24] SHAIKH M S, SHARIFF A M, BUSTAM M A, MURSHID G. Physicochemical properties of aqueous solutions of sodium glycinate in the non-precipitation regime from 298.15 to 343.15K[J]. Chin J Chem Eng, 2015, 23(3):536-540. doi: 10.1016/j.cjche.2013.11.001 [25] MAZINANI S, SAMSAMI A, JAHANMIRI A. Solubiity (at low partial pressures), density, viscosity, and corrosion rate of carbon dioxide in blend solutions of monoethanolamine (MEA) and sodium glycinate (SG)[J]. J Chem Eng Data, 2011, 56(7):3163-3168. doi: 10.1021/je2002418 [26] KUMARI A, SANDEEPA K, KUMAR T P, SATYAVATHI B. Solubility, thermodynamic properties, and derived excess properties of benzoic acid in (acetic acid + water) and (acetic acid + toluene) binary mixtures[J]. J Chem Eng Data, 2016, 61(1):67-77. doi: 10.1021/acs.jced.5b00197 [27] PHOON L Y, HASHIM H, MAT R, MUSTAFFA A A. Flash point prediction of tailor-made green diesel blends containing B5 palm oil biodiesel and alcohol[J]. Fuel, 2016, 175:287-293. doi: 10.1016/j.fuel.2016.02.027 [28] PRUGH R W. The relationship between flash point and LFL with application to hybrid mixtures[J]. Process Saf Prog, 2008, 27(2):156-163. doi: 10.1002/(ISSN)1547-5913 [29] PRAK D J L, COWART J S, TRULOVE P C. Density and viscosity from 293.15 to 373.15K, speed of sound and bulk modulus from 293.15 to 343.15 K, surface tension, and flash point of binary mixtures of bicyclohexyl and 1, 2, 3, 4-tetrahydronaphthalene or trans-decahydronaphthalene at 0.1MPa[J]. J Chem Eng Data, 2016, 61(1):650-661. doi: 10.1021/acs.jced.5b00790 [30] FLETCHER P D I, ROBERTS N A, URQUHART C. Solubility behavior, crystallization kinetics and pour point:A comparison of linear alkane and triacyl glyceride solute/solvent mixtures[J]. J Ind Eng Chem, 2016, 34:382-389. doi: 10.1016/j.jiec.2015.12.012 [31] GB 19147-2013, Automobile diesel fuels (V)[S]. [32] BRANDENBURG A, WAPPLER E, KITA J, MOOS R. Miniaturized ceramic DSC device with strain gauge-based mass detection-first steps to realize a fully integrated DSC/TGA device[J]. Sens Actuators A, 2016, 241:145-151. doi: 10.1016/j.sna.2016.02.011 [33] JIN X, XU X D, ZHANG X S, YIN Y G. Determination of the PCM melting temperature rang using DSC[J]. Thermochim Acta, 2014, 595:17-21. doi: 10.1016/j.tca.2014.09.004 -





 下载:
下载: