Preparation and application of a novel carbon-based solid acid with Brønsted-Lewis double acid sites for synthesis of biodiesel
-
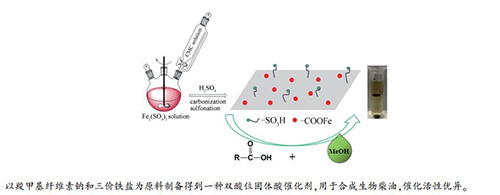
摘要: 以羧甲基纤维素钠(CMC)与硫酸铁螯合反应生成的螯合物为碳前驱体,以浓硫酸为磺化试剂,制备新型碳基固体酸催化剂。采用红外(FT-IR)、X射线衍射(XRD)、吡啶红外、扫描电子显微镜(SEM)、热重分析仪(TGA)、能谱仪(EDS)对催化剂进行表征。结果表明,该催化剂同时具有Brønsted和Lewis酸位点,是具有双酸位的碳基固体酸催化剂。将其应用到油酸与甲醇的酯化反应制备生物柴油体系中,考察了不同反应条件对油酸转化率的影响。在反应温度为70℃,反应时间为6h,油酸与甲醇物质的量比为1:10,催化剂用量为油酸质量7.5%条件下,油酸的转化率可达到96.8%。此外,对该催化剂的稳定性进行研究发现该催化剂有着良好的重复使用性和疏水性。Abstract: A novel carbon-based solid acid catalyst was prepared with chelates of sodium carboxymethyl cellulose and iron sulfate as a carbon precursor, and with concentrated sulfuric acid as sulfonation agent. The physical and chemical properties of prepared catalyst were characterized by Fourier transform infrared (FT-IR), X-ray diffraction (XRD), pyridine-FTIR, scanning electron microscopy (SEM), thermogravimetric analysis (TGA) and energy dispersive spectroscopy (EDS). It was used to catalyze the esterification of oleic acid with methanol to prepare biodiesel, and the influence of different reaction conditions on the conversion of oleic acid was investigated. The results show that the catalyst is a solid acid one with both Lewis and Brønsted acid sites. The conversion of oleic acid reaches 96.8% at the reaction temperature of 70℃, the reaction time of 6 h, the molar ratio of oleic acid to methanol of 1:10, and the catalyst dosages of 7.5% based on oleic acid. In addition, the stability performance of the catalyst tested indicates that the catalyst has a good reusability and hydrophobicity.
-
Key words:
- sodium carboxymethyl cellulose /
- sulfonation /
- double acid sites /
- biodiesel
-
表 1 Fe-CMC-SO3H催化剂不同元素的含量
Table 1 Content of different elements in Fe-CMC-SO3H catalyst
Sample Content w/% C O S Fe Fe-CMC-SO3H 55.71 42.71 1.38 0.2 Catalyst after fifth used 70.60 28.43 0.83 0.14 -
[1] HÖÖK M, TANG X. Depletion of fossil fuels and anthropogenic climate change:A review[J]. Energy Policy, 2013, 52:797-809. doi: 10.1016/j.enpol.2012.10.046 [2] WILSON K, LEE A F. Rational design of heterogeneous catalysts for biodiesel synthesis[J]. Catal Sci Technol, 2012, 2(5):884-897. doi: 10.1039/c2cy20038d [3] LEUNG D Y C, WU X, LEUNG M K H. A review on biodiesel production using catalyzed transesterification[J]. App Energy, 2010, 87(4):1083-1095. doi: 10.1016/j.apenergy.2009.10.006 [4] ATABANI A E, SILITONGA A S, BADRUDDIN I A, MAHLIA T M I, MEKHILEF M S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics[J]. Renewable Sustainable Energy Rev, 2012, 16(4):2070-2093. doi: 10.1016/j.rser.2012.01.003 [5] PINZI S, GARCIA I L, LOPEZ-GIMENEZ F J, LUQUE DE CASTRO M D, DORADO G, DORADO M P. The ideal vegetable oil-based biodiesel composition:A review of social, economical and technical implications[J]. Energy Fuels, 2009, 23(5):2325-2341. doi: 10.1021/ef801098a [6] DEMIRBAS A. Progress and recent trends in biodiesel fuels[J]. Energy Convers Manage, 2009, 50(1):14-34. doi: 10.1016/j.enconman.2008.09.001 [7] SU F, GUO Y. Advancements in solid acid catalysts for biodiesel production[J]. Green Chem, 2014, 16(6):2934-2957. doi: 10.1039/C3GC42333F [8] 王红红, 刘丽君, 龚树文.新型磷钨酸基固体酸催化油酸酯化合成生物柴油[J].燃料化学学报, 2017, 45(3):303-310. doi: 10.3969/j.issn.0253-2409.2017.03.007WANG Hong-hong, LIU Li-jun, GONG Shu-wen. Esterification of oleic acid to biodiesel over a 12-phosphotungstic acid-based solid catalyst[J]. J Fuel Chem Technol, 2017, 45(3):303-310. doi: 10.3969/j.issn.0253-2409.2017.03.007 [9] SHIBASAKI-KITAKAWA N, HONDA H, KURIBAYASHI H, TODA T, FUKUMURA T, YONEMOTO T. Biodiesel production using anionic ion-exchange resin as heterogeneous catalyst[J]. Bioresour Technol, 2007, 98(2):416-421. doi: 10.1016/j.biortech.2005.12.010 [10] MARGOLESE D, MELERO J A, CHRISTIANSEN S C, CHMELKA B F, STUCKY G D. Direct syntheses of ordered SBA-15 mesoporous silica containing sulfonic acid groups[J]. Chem Mater, 2000, 12(8):2448-2459. doi: 10.1021/cm0010304 [11] 李梦天, 蒋平平, 张萍波.碳基固体酸催化剂的制备及其催化油酸甲酯合成[J].精细化工, 2018, 35(4):638-644. http://d.old.wanfangdata.com.cn/Periodical/jxhg201804017LI Meng-tian, JIANG Ping-ping, ZHANG Ping-bo. Catalytic synthesis of methyl oleate by carbonbased solid acid[J]. Fine Chem, 2018, 35(4):638-644. http://d.old.wanfangdata.com.cn/Periodical/jxhg201804017 [12] 娄文勇, 蔡俊, 段章群, 宗敏华.基于纤维素的固体酸催化剂的制备及其催化高酸值废油脂生产生物柴油[J].催化学报, 2011, 32(5):1755-1761. http://d.old.wanfangdata.com.cn/Periodical/cuihuaxb201111013LOU Wen-yong, CAI Jun, DUAN Zhang-qun, ZONG Min-hua. Preparation of cellulose-derived solid acid catalyst and its use for production of biodiesel from waste oils with high acid value[J]. Chin J Catal, 2011, 32(5):1755-1761. http://d.old.wanfangdata.com.cn/Periodical/cuihuaxb201111013 [13] ZENG D L, LIU S L, GONG W J, WANG G H, QIU J H, CHEN H X. Synthesis, characterization and acid catalysis of solid acid from peanut shell[J]. Appl Catal A:Gen, 2014, 469:284-289. doi: 10.1016/j.apcata.2013.09.038 [14] 沈忠权, 余锡孟, 陈纪忠.新型磺化竹炭材料催化酯化反应[J].化工学报, 2015, 66(8):3072-3077. http://d.old.wanfangdata.com.cn/Periodical/hgxb201508042SHEN Zhong-quan, YU Xi-meng, CHEN Ji-zhong.Esterification reactions catalyzed by novel sulfonated carbon material derived from bamboo[J]. J Chem Ind Eng, 2015, 66(8):3072-3077. http://d.old.wanfangdata.com.cn/Periodical/hgxb201508042 [15] 牛生洋, 郝峰鸽.羧甲基纤维素钠的应用进展[J].安徽农业科学, 2006, 34(15):3574-3575. doi: 10.3969/j.issn.0517-6611.2006.15.005NIU Sheng-yang, SHAO Feng-ge. Application progress in the carboxymethyl cellulose sodium[J]. J Anhui Agri Sci, 2006, 34(15):3574-3575. doi: 10.3969/j.issn.0517-6611.2006.15.005 [16] BISWALl D R, SINGH R P. Characterisation of carboxymethyl cellulose and polyacrylamide graft copolymer[J]. Carbohydr Polym, 2004, 57(4):379-387. doi: 10.1016/j.carbpol.2004.04.020 [17] WANG Y, WANG D, TAN M H, JIANG B, ZHENG J T, TSUBAKI N, WU M B. Monodispersed hollow SO3 H-functionalized carbon/silica as efficient solid acid catalyst for esterification of oleic acid[J]. ACS Appl Mater Interfaces, 2015, 7(48):26767-26775. doi: 10.1021/acsami.5b08797 [18] WANG Y T, FANG Z, YANG X X. Biodiesel production from high acid value oils with a highly active and stable bifunctional magnetic acid[J]. App Energy, 2017, 204:702-714. doi: 10.1016/j.apenergy.2017.07.060 [19] YU J T, DEHKHODA A M, ELLIS N. Development of biochar-based catalyst for transesterification of canola oil[J]. Energy Fuels, 2010, 25(1):337-344. doi: 10.1021/ef100977d [20] LIU H, CHEN J Z, CHEN L M, XU Y S, GUO X H, FANG D Y. Carbon nanotube-based solid Sulfonic acids as catalysts for production of fatty acid methyl ester via transesterification and esterification[J]. ACS Sustainable Chem Eng, 2016, 4(6):3140-3150. doi: 10.1021/acssuschemeng.6b00156 [21] ZONG M H, DUAN Z Q, LOU W Y, SMITH T J, WU H. Preparation of a sugar catalyst and its use for highly efficient production of biodiesel[J]. Green Chem, 2007, 9(5):434-437. doi: 10.1039/b615447f [22] EMEIS C A. Determination of integrated molar extinction coefficients for infrared absorption bands of pyridine adsorbed on solid acid catalysts[J]. J Catal, 1993, 141(2):347-354. doi: 10.1006-jcat.1993.1145/ [23] 朱烨楠, 马田林, 丁建飞.硫酸改性MCM-41分子筛催化剂的制备及其在甘油脱水制备丙烯醛中的应用[J].合成化学, 2016, 24(1):67-70. http://d.old.wanfangdata.com.cn/Periodical/hchx201601016ZHU Ye-nan, MA Tian-lin, DING Jian-fei. Preparation of H2SO4/MCM-41 catalyst and their application in dehydration of glycerol into acrolein[J]. Chin J Synth Chem, 2016, 24(1):67-70. http://d.old.wanfangdata.com.cn/Periodical/hchx201601016 [24] SHU Q, TANG G Q, LESMANA H, ZOU L X, XIONG D L. Preparation, characterization and application of a novel solid Brønsted acid catalyst SO42-/La3+/C for biodiesel production via esterification of oleic acid and methanol[J]. Renewable Energy, 2018, 119:253-261. doi: 10.1016/j.renene.2017.12.013 [25] MALINS K, KAMPARS V, BRINKS J, NEIBOLTE L, MURNIEKS R. Synthesis of activated carbon based heterogenous acid catalyst for biodiesel preparation[J]. App Catal B:Environ, 2015, 176:553-558. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=eda0736b3854e61942e9c1148fb44fd5 -





 下载:
下载: