Reaction and hydrogen transfer in complex multi-phase system during coal hydro-liquefaction
-
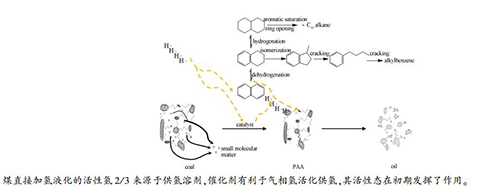
摘要: 研究新疆淖毛湖煤(NMH)在四氢萘为溶剂条件下的加氢液化反应行为,探究了液化过程氢传递规律,并借助XRD、饱和磁化强度和扫描电镜表征手段,研究了煤液化条件下铁系催化剂的相态变化对煤液化性能的影响。结果表明,NMH煤在420℃、17 MPa就具有良好的液化效果;催化剂的活性态Fe7S8在煤液化反应初期发挥了催化作用,加氢液化后期,转变为非活性态Fe9S10和FeS;提高催化剂加氢活性并延长反应时间有利于沥青烯和前沥青烯加氢轻质化;催化剂有利于活化气相氢向煤的热解产物和溶剂转移,也有利于活化溶剂中的氢向煤的热解产物转移;溶剂对液化反应的活性氢贡献更大,约为气相氢的两倍,气相氢向溶剂传递的氢量随温度的升高、压力的增大和时间的延长变化不大,气相氢和供氢溶剂供氢与煤和沥青质向油气转化呈正相关。Abstract: Reaction behavior of Naomaohu (NMH) coal in tetralin was carried out under atmosphere of H2. Hydrogen transfer in complex multi-phase system of direct coal liquefaction were discussed. The influence of phase transition process of iron-based catalyst on liquefaction performance was investigated using X-ray diffraction, saturate magnetization and scanning electron microscope. The results show that NMH coal presents good liquefaction performance at 420℃ and 17 MPa. Active phase Fe7S8 plays catalytic role during initial reaction and changes into nonactive phase-Fe9S10 and FeS later. High hydrogenation activity of catalyst and long residence time are beneficial to hydrogenation of preasphaltene and asphaltene into light oil. Catalyst promotes the activation of H2 transferring to coal pyrolysis products and solvent. Catalyst promotes the hydrogen in solvent to transfer to coal pyrolysis products as well. The contribution to activated hydrogen from solvent is twice as that from H2 in the condition of the experiment. Hydrogen transferring from H2 to solvent changes little with temperature, pressure and time. Activated hydrogen from H2 and solvent is proportional to the conversion of coal and asphaltene to oil and gas.
-
Key words:
- low rank coal /
- hydroliquefaction /
- hydrogen transfer process /
- catalysis activity
-
表 1 新疆淖毛湖煤质分析数据
Table 1 Analysis data of NMH coal
Ultimate analysis wdaf/% C H N S O 74.97 5.16 1.00 0.20 18.67 Proximate analysis w/% Petrographic analysis φ/% Ad Vdaf V I E M 5.12 49.69 88.8 0.4 7.6 3.2 表 2 不同条件下淖毛湖煤加氢液化实验数据
Table 2 NMH coal liquefaction results under different conditions
No. Cat. t/℃ p/MPa Time t/min Conv. x/% O w/% PAA w/% G w/% 1# Fe2O3 440 7.5 60 97.25 70.75 1.13 16.30 2# Fe2O3 420 7.5 0 91.23 56.06 15.85 9.46 3# Fe2O3 420 7.5 30 94.11 65.63 6.74 12.27 4# Fe2O3 420 9.5 60 94.12 68.01 2.55 13.77 5# Fe2O3 420 7.5 60 94.14 67.91 3.57 13.23 6# Mo-Fe 420 7.5 60 95.05 68.56 3.05 13.53 7# - 420 7.5 60 87.87 60.04 5.40 12.01 8# Fe2O3 420 5.5 60 93.66 65.45 6.52 12.53 9# Fe2O3 420 7.5 90 93.85 67.22 3.77 14.17 10# Fe2O3 400 7.5 60 86.20 53.81 12.32 10.06 11# Fe2O3 380 7.5 60 82.55 48.21 10.45 8.89 表 3 不同时间Fe的XRD和饱和磁化强度
Table 3 XRD and saturation magnetization under different times
Compound Crystal Time t/min Atmosphere 0 30 60 90 N2 H2 Fe7S8 monoclinic ■ ■ ■ ■ ■ ■ triclinic ■ ■ ■ ■ ■ ■ Fe9S10 hexagonal - ■ ■ ■ ■ ■ Fe0.95S hexagonal ■ ■ ■ ■ ■ ■ FeS hexagonal ■ ■ ■ ■ ■ ■ Fe2O3 cubic ■ ■ ■ ■ ■ ■ Fe3O4 - ■ ■ ■ - ■ ■ Ms/(emu·g) 0.61 0.41 0.24 0.09 0.18 0.24 ■:detected; -:not detected 表 4 四氢萘的加氢液化产物组成
Table 4 Composition of tetralin hydroliquefaction product
Compound name Toluene Decalin Tetralin Naphthalene Content w/% 2.98 0.5 78.57 12.92 Compound name 1-methylindan butylbenzene n-heptane Content w/% 1.81 1.26 1.95 -
[1] 刘振宇.煤直接液化技术发展的化学脉络及化学工程挑战[J].化工进展, 2010, 29(2):193-195. http://d.old.wanfangdata.com.cn/Periodical/hgjz201002001LIU Zhen-yu. Principal chemistry and chemical engineering challenges in direct coal liquefaction technology[J]. Chem Ind Eng Prog, 2010, 29(2):193-195. http://d.old.wanfangdata.com.cn/Periodical/hgjz201002001 [2] ZIELKE C W, STRUCK R T, EVANS J M, COSTANZA C P, GORIN E. Molten zinc halide catalysts for hydrocracking of coal extract and coal[J]. Ind Eng Chem Process Des Dev, 1966, 5(2):158-164. doi: 10.1021/i260018a009 [3] CURRAN G P, STRUCK R T, GORIN E. Mechanism of hydrogen-transfer process to coal and coal extract[J]. Ind Eng Chem Process Des Dev, 1967, 6(2):166-173. doi: 10.1021/i260022a003 [4] IKENAGA N, KAN-NAN S, SAKODA T, SUZUKI T. Coal hydroliquefaction using highly dispersed catalyst precursors[J]. Catal Today, 1997, 39(1/2):99-109. http://www.sciencedirect.com/science/article/pii/S0920586197000928 [5] GODO M, SAITO M, ISHIHAVE A. Elucidation of coal liquefaction mechanisms using a tritiated molecular hydrogen in the presence and absence of H2S[J]. Fuel, 1998, 77(9/10):947-952. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=0cd16ac2f4ab5001e9d718e5a37132ef [6] MCMILLEN D F, MALHOTRA R, HUM G P, CHANG S J. Hydrogen-transfer-promoted bond scission initiated by coal fragments[J]. Energy Fuels, 1987, 1(2):193-198. doi: 10.1021/ef00002a009 [7] MCMILLEN D F, MALHOTRA R, CHANG S J, OGIER W C, NIGENDA S E. Mechanisms of hydrogen transfer and bond scission of strongly bonded coal structures in donor-solvent systems[J]. Fuel, 1987, 66(12):1611-1620. doi: 10.1016/0016-2361(87)90351-6 [8] MALHOTRA R, MCMILLEN D F, TSE D S, LORENTS D C, RUOFF R S. Hydrogen-transfer reactions catalyzed by fullerenes[J]. Energy Fuels, 1993, 7(5):685-686. doi: 10.1021/ef00041a020 [9] VERNON L W. Free radical chemistry of coal liquefaction:Role of molecular hydrogen[J]. Fuel, 1980, 59(2):102-106. doi: 10.1016/0016-2361(80)90049-6 [10] ZONG Z M, WEI X Y. Effects of molecular hydrogen and hydrogen donor additives on 1, 2-di (1-naphthyl) ethane thermolysis[J]. Fuel Process Technol, 1994, 41(1):79-85. doi: 10.1016/0378-3820(94)90061-2 [11] KUHLMANN E J, JUNG D Y, GUPTILL R P, DYKE C A, ZANG H K. Coal liquefaction using a hydrogenated creosote oil solvent:H-atom transfer from hydrogen donor components in the solvent[J]. Fuel, 1985, 64(11):1552-1557. doi: 10.1016/0016-2361(85)90372-2 [12] DEPEYRE D, URHAN M, FLICOTEAUX C. Pyrolysis of hydrocarbon mixtures characteristic of coal:Application to dibenzyl mixture[J]. Fuel, 1985, 64(12):1655-1661. doi: 10.1016/0016-2361(85)90389-8 [13] CRONAUER D C, SHAH Y T, RUBERTO R G. Mechanism and kinetics of selected hydrogen transfer reactions typical of coal Liquefaction[J]. Ind Eng Chem Process Des Dev, 1978, 17:281. doi: 10.1021/i260067a013 [14] LI X, HU S X, JIN L, HU H Q. Role of iron-based catalyst and hydrogen transfer in direct coal liquefaction[J]. Energy Fuels, 2008, 22(2):1126-1129. doi: 10.1021/ef7006062 [15] 王建友.神华煤直接液化的催化加氢反应特性研究[D].大连: 大连理工大学, 2013: 81-85.WANG Jian-you. Characteristics of catalytic-hydrogenation in direct Shenhua coal liquefaction[D]. Dalian: Dalian University of Technology, 2013: 81-85. [16] 舒歌平.煤炭液化技术[M].北京:煤炭工业出版社, 2003:91-95.SHU Ge-ping. Direct Coal Liquefaction[M]. Beijing:Coal Industry Press, 2003:91-95. [17] 史士东.煤加氢液化工程学基础[M].北京:化学工业出版社, 2012:224-231.SHI Shi-dong. Engineering Fundamentals of Direct Coal Liquefaction[M]. Beijing:Chemical Industry Press, 2012:224-231. [18] KOTANIGAWA T, YOKOYAMA S, YAMAMOTO M, MAEKAWA Y. Catalytic activities of sulphate and sulphide in sulphur-promoted iron oxide catalyst for coal liquefaction[J]. Fuel, 1989, 68:618-621. doi: 10.1016/0016-2361(89)90161-0 -





 下载:
下载: