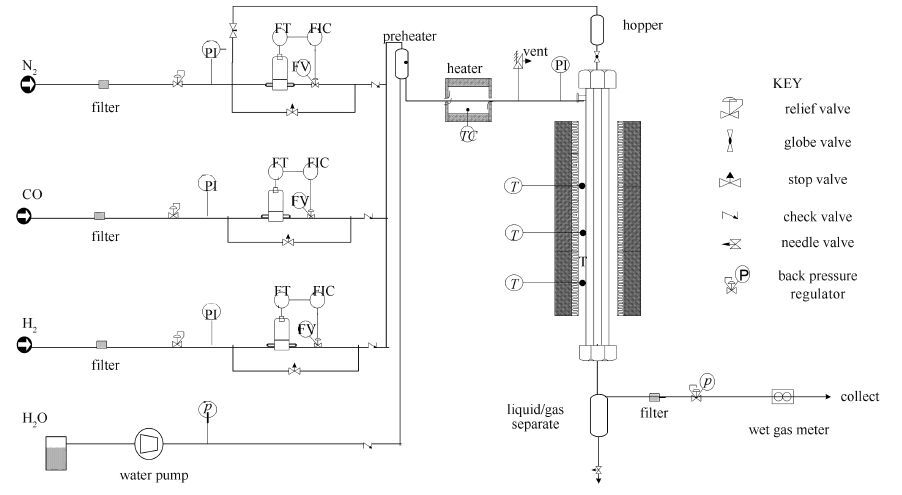
-
摘要: 以不连沟次烟煤为研究对象,在加压固定床中研究Ca(OH)2对煤气化及负载Ca(OH)2煤热解半焦的甲烷化反应活性。结果表明,煤中添加Ca(OH)2能够明显促进气化反应的进行和甲烷的生成,随着气化温度的升高和负载量的增加,碳转化率增加,但负载量存在饱和点。不同的催化剂负载方式对Ca的分散有一定的影响,进而影响其催化性能。含有Ca的半焦能够明显促进甲烷化的进行,出口气甲烷含量随甲烷化温度和催化剂负载量的升高而增强。采用红外光谱分析揭示了煤负载Ca(OH)2的离子交换机理和扩散过程,这一过程影响煤气化反应性能。Abstract: The catalytic gasification performance of Buliangou coal using Ca(OH)2 as catalyst was investigated at 3.5 MPa and 700-800℃ in a pressurized fixed bed. The effect of gasification temperature, Ca(OH)2 loading and loading method on coal steam gasification and methanation reaction were examined. The results show that Ca(OH)2 can enhance the reactivity of char gasification and the formation of CH4. Increasing temperature and Ca(OH)2 loading can heighten the carbon conversion, but Ca(OH)2 loading possesses a saturation point. The gasification reactivity is affected by loading method which determines the Ca(OH)2 dispersion. The coal char loaded with Ca(OH)2 shows a great catalytic activity on methanation reaction and the CH4 content in the product gas increases with the increase of methanation temperature and catalyst loading. The analysis result of coal surface function groups by FT-IR reveals the dispersion mechanism of Ca(OH)2 into the matrix of coal through ion exchange and diffusion, which is a key factor to improve coal char gasification reactivity.
-
Key words:
- catalytic gasification /
- Ca(OH)2 /
- methanation /
- coal char
-
表 1 煤样的工业分析及元素分析
Table 1 Proximate and ultimate analyses of coal sample
Proximate analysis wad/% Ultimate analysis (dry and ash free basis ) wdaf/% M A V FC C H Oa N S 2.76 19.02 28.65 49.57 77.55 4.58 15.49 1.36 1.02 a : by difference 表 2 灰组成分析
Table 2 Ash composition
Composition of ash w/% SiO2 Al2O3 Fe2O3 CaO MgO TiO2 SO3 P2O3 /p>K2O Na2O 28.01 48.15 7.13 9.11 1.09 1.99 2.83 0.45 0.27 0.28 ash fusion point: DT = 1 447 ℃, ST > 1 500 ℃, HT > 1 500 ℃, FT > 1 500 ℃ -
[1] 陈玉爽, 张忠孝, 乌晓江, 李洁, 管嵘清, 闫博.配煤对煤灰熔融特性影响的实验与量化研究[J].燃料化学学报, 2009, 37(5):521-526. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract17485.shtmlCHEN Yu-shuang, ZHANG Zhong-xiao, WU Xiao-jiang, LI Jie, GUAN Rong-qing, YAN Bo.Quantum chemistry calculation and experimental study on coal ash fusion characteristics of blend coal[J].J Fuel Chem Technol, 2009, 37(5):521-526. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract17485.shtml [2] HIRSCH R L, GALLAGHER J E, LESSARD R R, WESSELHOFT R D.Catalytic coal gasification:An emerging technology[J].Science, 1982, 215(4529):121-127. doi: 10.1126/science.215.4529.121 [3] GreatPoint Energy, Hydromethanation via Bluegas Technology[DB].<http://www.greatpointenergy.com/about.php> [4] LI W W, LI K Z, QU X, ZHANG R, BI J C.Simulation of catalytic coal gasification in a pressurized jetting fluidized bed:Effects of operating conditions[J].Fuel Process Technol, 2014, 126:504-512. doi: 10.1016/j.fuproc.2014.06.006 [5] GALLGHER J E, EUKER C A.Catalytic coal gasification for SNG manufacture[J].Energy Res, 1980, 4:137-147. doi: 10.1002/(ISSN)1099-114X [6] FORMELLA K, LEONHARDT P, SULIMMA A, VAN HEEK K H, JVNTGEN H.Interaction of mineral matter in coal with potassium during gasification[J].Fuel, 1986, 65(10):1470-1472. doi: 10.1016/0016-2361(86)90126-2 [7] BL SING M, MVLLER M.Investigations on the influence of steam on the release of sodium, potassium, chlorine, and sulphur species during high temperature gasification of coal[J].Fuel, 2012, 94:137-143. doi: 10.1016/j.fuel.2011.11.052 [8] 毛燕东, 金亚丹, 王会芳, 郑岩, 李克忠, 毕继诚, 李金来, 辛峰.煤催化气化工艺中碱金属腐蚀刚玉质耐火材料的实验研究.燃料化学学报, 2014, 42(11):1332-1339. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18522.shtmlMAO Yan-dong, JIN Ya-dan, WANG Hui-fang, ZHENG Yan, LI Ke-zhong, BI Ji-cheng, LI Jin-lai, XIN Feng.Experimental research on corrosions of corundum refractory by alkali metals in catalytic coal gasification process[J].J Fuel Chem Technol, 2014, 42(11):1332-1339. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18522.shtml [9] OHTSUKA Y, TOMITA A.Calcium catalysed steam gasification of Yallourn brown coal[J].Fuel, 1986, 65(12):1653-1657. doi: 10.1016/0016-2361(86)90264-4 [10] OHTSUKA Y, ASAMI K.Steam gasification of coals with calcium hydroxide[J].Energy Fuels, 1995, 9(6):1038-1042. doi: 10.1021/ef00054a016 [11] ZHANG Y, ASHIZAWA M, KAJITANI S.Calcium loading during the dewatering of wet biomass in kerosene and catalytic activity for subsequent char gasification[J].Fuel, 2008, 87(13/14):3024-3030. [12] ZHANG Y, ASHIZAWA M, KAJITANI S, HARA S.A new approach to catalytic coal gasification:The recovery and reuse of calcium using biomass derived crude vinegars[J].Fuel, 2010, 89(2):417-422. doi: 10.1016/j.fuel.2009.07.009 [13] OTTO K, BARTOSIEWICZ L, SHELEF M.Effects of calcium, strontium, and barium as catalysts and sulphur scavengers in the steam gasification of coal chars[J].Fuel, 1979, 58(8):565-572. doi: 10.1016/0016-2361(79)90004-8 [14] RADOVIC L R, WALKER P L, JENKINS R G.Importance of catalyst dispersion in the gasification of lignite chars[J].J Catal, 1983, 82(2):382-394. doi: 10.1016/0021-9517(83)90205-1 [15] RADOVIC L A.Catalysis in Coal and Carbon Gasification[C]//Handbook of heterogeneous catalysis.Weinheim:Wiley-VCH Verlag GmbH&Co.KGa A.2008, 3040. [16] 赵明举, 谢克昌, 凌大琦.煤中矿物质在煤气化中的作用[J].煤炭转化, 1989, 1:23-19. http://www.cnki.com.cn/Article/CJFDTOTAL-MTZH198901003.htmZHAO Ming-ju, XIE Ke-chang, LING Da-qi.Effect of coal mineral on coal gasification[J].Coal Convers, 1989, 1:23-19. http://www.cnki.com.cn/Article/CJFDTOTAL-MTZH198901003.htm [17] CORELLA J, TOLEDO J M, MOLINA G.Steam gasification of coal at low-medium (600-800℃) temperature with simultaneous CO2 capture in fluidized bed at atmospheric pressure:The effect of inorganic species.1.literature review and comments[J].Ind Eng Chem Res, 2006, 45(18):6137-6146. doi: 10.1021/ie0602658 [18] SPIRO C L, MCKEE D W, KOSKY P G, LAMBY E J.Observation of alkali catalyst particles during gasification of carbonaceous materials in CO2 and steam[J].Fuel, 1984, 63(5):686-691. doi: 10.1016/0016-2361(84)90167-4 [19] MATSUKATA M, KIKUCHI E, MORITA Y.A new classification of alkali and alkaline earth catalysts for gasification of carbon[J].Fuel, 1992, 71(7):819-823. doi: 10.1016/0016-2361(92)90136-C [20] LIN S Y, HARADA M, SUZUKI Y, HATANO H.Continuous experiment regarding hydrogen production by Coal/CaO reaction with steam (I) gas products[J].Fuel, 2004, 83(7/8):869-874. [21] LIN S Y, HARADA M, SUZUKI Y, HATANO H.Continuous experiment regarding hydrogen production by Coal/CaO reaction with steam (Ⅱ) solid formation[J].Fuel, 2006, 85(7/8):1143-1150. [22] NAHAS N C.Exxon catalytic coal gasification process:Fundamentals to flowsheets[J].Fuel, 1983, 62(2):239-241. doi: 10.1016/0016-2361(83)90207-7 [23] OTAKE T, TONE S, KIMURA S, HINO Y.Methane formation over potassium carbonate catalyst loaded on coal char[J].J Chem Eng Japan, 1984, 17(5):503-507. doi: 10.1252/jcej.17.503 [24] MEIJER R, VAN DOORN R, KAPTEIJN F, MOULIJN J A.Methane formation in H2, CO mixtures over carbon-supported potassium carbonate[J].J Catal, 1992, 134(2):525-535. doi: 10.1016/0021-9517(92)90339-J [25] 樊利霞, 李克忠, 张荣, 毕继诚.负载碳酸钾煤焦上CO甲烷化反应的研究[J].燃料化学学报, 2014, 42(9):1047-1052. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18483.shtmlFAN Li-xia, LI Ke-zhong, ZHANG Rong, BI Ji-cheng.Methanation of CO over coal char loaded with K2CO3[J].J Fuel Chem Technol, 2014, 42(9):1047-1052. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18483.shtml [26] CASANOVA R, CABRERA A L, HEINEMANN H, SOMORJAI G A.Calcium oxide and potassium hydroxide catalysed low temperature methane production from graphite and water comparison of catalytic mechanisms[J].Fuel, 1983, 62(10):1138-1144. doi: 10.1016/0016-2361(83)90053-4 [27] 郑庆荣, 曾凡桂, 张世同.中变质煤结构演化的FT-IR分析[J].煤炭学报, 2011, 36(3):481-486. http://www.cnki.com.cn/Article/CJFDTOTAL-MTXB201103025.htmZHENG Qing-rong, ZENG Fan-gui, ZHANG Shi-tong.FT-IR study on structure evolution of middle maturate coals[J].J China Coal Soc, 2011, 36(3):481-486. http://www.cnki.com.cn/Article/CJFDTOTAL-MTXB201103025.htm [28] OHTSUKA Y, ASAMI K.Ion-exchanged calcium from calcium carbonate and low-rank coals:High catalytic activity in steam gasification[J].Energy Fuels, 1996, 10(2):431-435. doi: 10.1021/ef950174f [29] OHTSUKA Y, ASARNI K.Highly active catalysts from inexpensive raw materials for coal gasification[J].Catal Today, 1997, 39(1/2):111-125. [30] ILLáN-GóMEZ M J, GARCíA-GARCíA A, LECEA C, LINARES-SOLANO A.Activated carbons from spanish coals.2.Chemical activation[J].Energy Fuels, 1996, 10(5):1108-1114. doi: 10.1021/ef950195+ -





 下载:
下载: