Effect of calcination temperature on catalytic performance of Pt-FeOx/γ-Al2O3 catalysts for HCHO oxidation
-
摘要: 采用胶体沉积法制备了Pt-FeOx/γ-Al2O3催化剂,通过XRD、TEM、BET、XPS、H2-TPR和FT-IR等技术对催化剂进行了表征,考察了焙烧温度对Pt-FeOx/γ-Al2O3催化剂表面结构及其催化甲醛氧化性能的影响。结果表明,焙烧温度对Pt-FeOx/γ-Al2O3催化剂的氧化还原性能、Pt物种的化学状态以及表面羟基的数量有较大的影响。在室温下,所有Pt-FeOx/γ-Al2O3催化剂均表现出催化氧化活性,其中,200℃焙烧的Pt-FeOx/γ-Al2O3催化剂表现出最好的催化性能,可以将甲醛100%转化为CO2和H2O。较低温度焙烧的Pt-FeOx/γ-Al2O3催化剂表面Pt物种具有较好的价态分布以及更多的界面活性位,如Pt-O-Fe物种,因而在温和条件下对甲醛的催化氧化活性较高。Abstract: A series of Pt-FeOx/γ-Al2O3 catalysts were prepared by colloid-deposition method and characterized by XRD, TEM, BET, XPS, H2-TPR and FT-IR to investigate the effects of calcination temperature on the surface structure of Pt-FeOx/γ-Al2O3 catalyst and its catalytic performance in catalytic HCHO oxidation. The characterization results showed that the applied calcination temperature greatly influenced the redox properties and chemical states of the Pt species, as well as the amount of surface hydroxyl groups. All resultant Pt-FeOx/γ-Al2O3 catalysts demonstrated activity in HCHO oxidation. The sample with calcination at 200 ℃ exhibited the best performance, which afforded 100% conversion of HCHO into CO2 and H2O at room temperature. The catalysts with lower calcination temperature should be beneficial to have a better valence distribution of Pt species and produce more accessible interface active sites like Pt-O-Fe species, thus endowing Pt-FeOx/γ-Al2O3 catalyst with relatively high activity for the oxidation of formaldehyde under mild conditions.
-
Key words:
- formaldehyde /
- catalytic oxidation /
- reactivity /
- calcination temperature /
- Pt nanoparticles
-
图 3 t/γ-Al2O3-200和Pt-FeOx/γ-Al2O3-t催化剂的HRTEM电镜照片((a)-(d), (f)),Pt-FeOx/γ-Al2O3-200催化剂的HAADF-STEM电镜照片(e)和EDX元素能量分布图(g)
Figure 3 HRTEM ((a)-(d), (f)) of the Pt/γ-Al2O3-200 and Pt-FeOx/γ-Al2O3 -t catalyst, HAADF-STEM(e) and EDX mapping images(g) of the Pt-FeOx/γ-Al2O3-200 catalyst
(a): Pt/γ-Al2O3-200; (b): Pt-FeOx/γ-Al2O3-200; (c): Pt-FeOx/γ-Al2O3-300; (d): Pt-FeOx/γ-Al2O3-400 (e): Pt-FeOx/γ-Al2O3-200; (f): Pt-FeOx/γ-Al2O3-200; (g): Pt-FeOx/γ-Al2O3-200
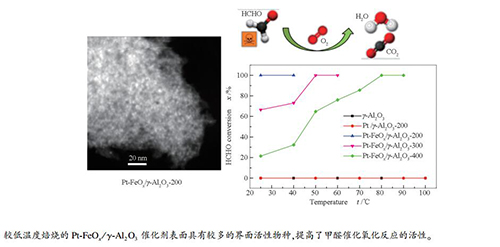
图 4 Pt/γ-Al2O3-200、Pt-FeOx/γ-Al2O3-t催化剂和γ-Al2O3载体的甲醛催化氧化活性
Figure 4 HCHO catalytic oxidation activities of the Pt/γ- Al2O3-200, Pt-FeOx/Al2O3-t catalysts and γ-Al2O3 support
a: γ-Al2O3; b: Pt/γ-Al2O3-200; c: Pt-FeOx/γ-Al2O3-200; d: Pt-FeOx/γ-Al2O3-300; e: Pt-FeOx/γ-Al2O3-400 (reaction conditions: HCHO 375 mg/m3, φO2=20%, N2 balance, RH = 30%, GHSV: 60000 cm3/(g·h))
表 1 Pt/γ-Al2O3-200、Pt-FeOx/γ-Al2O3-t催化剂和γ-Al2O3载体的物化性能
Table 1 Physical properties of the Pt/γ-Al2O3-200, Pt-FeOx/Al2O3-t catalysts and γ-Al2O3 support
Catalyst ABET/(m2·g-1) Pore volume v/(m3·g-1) Pore size d/nm γ-Al2O3 162 0.23 6.0 Pt/γ-Al2O3-200 119 0.17 6.5 Pt-FeOx/γ-Al2O3-200 113 0.17 6.6 Pt-FeOx/γ-Al2O3-300 104 0.16 6.7 Pt-FeOx/γ-Al2O3-400 98 0.14 6.9 表 2 Pt/γ-Al2O3-200、Pt-FeOx/γ-Al2O3-t催化剂以及γ-Al2O3载体的Pt 4d和O 1s XPS拟合
Table 2 Pt 4d and O 1s XPS curve fitting results of Pt/γ- Al2O3-200, Pt-FeOx/Al2O3-t catalysts and γ-Al2O3 support
Catalyst Pt2+/Pt 0 OII/(OI+ OII) γ-Al2O3 0.37 Pt/γ-Al2O3-200 0.36 0.31 Pt-FeOx/γ-Al2O3-200 0.39 0.31 Pt-FeOx/γ-Al2O3-300 0.58 0.23 Pt-FeOx/γ-Al2O3-400 1.33 0.18 -
[1] SALTHAMMER T, MENTESE S, MARUTZKY R. Formaldehyde in the indoor environment[J]. Chem Rev, 2010, 110(4):2536-2572. doi: 10.1021/cr800399g [2] CHI C C, CHEN W D, GUO M, WENG M L, YAN G, SHEN X Y. Law and features of TVOC and formaldehyde pollution in urban indoor air[J]. Atmos Environ, 2016, 132(5):85-90. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=1521901c1510a13ac0d0c9c6162c34fb [3] TANG X J, BAI Y, DUONG A, SMITH M T, LI L Y, ZHANG L P. Formaldehyde in China:Production, consumption, exposure levels, and health effects[J]. Environ Int, 2009, 35(8):1210-1224. doi: 10.1016/j.envint.2009.06.002 [4] MARSH G M, YOUK A O. Reevaluation of mortality risks from nasopharyngeal cancer in the formaldehyde cohort study of the national cancer institute[J]. Regul Toxicol Pharmacol, 2004, 40(11):113-124. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=7a3fdf4cc193217c05653b2e7b70f81d [5] HUANG H B, XU Y, FENG Q Y, LEUNG D Y C. Low temperature catalytic oxidation of volatile organic compounds:A review[J]. Catal Sci Technol, 2015, 5(2):2649-2669. http://www.mendeley.com/research/low-temperature-catalytic-oxidation-volatile-organic-compounds-review/ [6] NIE L H, YU J G, JARONIEC M, TAO F. Room-temperature catalytic oxidation of formaldehyde on catalysts[J]. Catal Sci Technol, 2016, 6(11):3649-3669. doi: 10.1039/C6CY00062B [7] 拜冰阳, 乔琦, 李俊华, 郝吉明.甲醛催化氧化催化剂的研究进展[J].催化学报, 2016, 37(1):102-122. http://d.old.wanfangdata.com.cn/Periodical/cuihuaxb201601013BAI Bing-yang, QIAO Qi, LI Jun-hua, HAO Ji-ming. Progress in research on catalysts for catalytic oxidation of formaldehyde[J]. Chin J Catal, 2016, 37(1):102-122. http://d.old.wanfangdata.com.cn/Periodical/cuihuaxb201601013 [8] ZHANG C B, LIU F D, ZHAI Y P, ARIGA H, YI N, LIU Y C, ASAKURA K, FLYTZANI-STEPHANOPOULOS M, HE H. Alkali-metal-promoted Pt/TiO2 opens a more efficient pathway to formaldehyde oxidation at ambient temperatures[J]. Angew Chem Int Ed, 2012, 51(38):9628-9632. doi: 10.1002/anie.v51.38 [9] XU Q L, LEI W Y, LI X Y, QI X Y, YU J G, LIU G, WANG J L, ZHANG P Y. Efficient removal of formaldehyde by nanosized gold on well-defined CeO2 nanorods at room temperature[J]. Environ Sci Technol, 2014, 48(16):9702-9708. doi: 10.1021/es5019477 [10] LI Y B, ZHANG C B, HE H, ZHANG J H, CHEN M. Influence of alkali metals on Pd/TiO2 catalysts for catalytic oxidation of formaldehyde at room temperature[J]. Catal Sci Technol, 2016, 6(7):2289-2295. doi: 10.1039/C5CY01521A [11] MA L, WANG D S, LI J H, BAI B Y, FU L X, LI Y D. Ag/CeO2 nanospheres:Efficient catalysts for formaldehyde oxidation[J]. Appl Catal B:Environ, 2014, 148/149(7):36-43. http://www.sciencedirect.com/science/article/pii/S0926337313006644 [12] WANG J L, LI J G, JIANG C J, ZHOU P, ZHANG P Y, YU J G. The effect of manganese vacancy in birnessite-type MnO2 on room-temperature oxidation of formaldehyde in air[J]. Appl Catal B:Environ, 2017, 204(5):147-155. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=fb70db68f2265617d095cbbae17d7b14 [13] XIA Y S, DAI H X, ZHANG L, DENG J G, HE H, AU C T. Ultrasound-assisted nanocasting fabrication and excellent catalytic performance of three-dimensionally ordered mesoporous chromia for the combustion of formaldehyde, acetone, and methanol[J]. Appl Catal B:Environ, 2010, 100(10):229-237. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=5314897f591d3c6b2316eedcef2668cf [14] WANG Z, WANG W Z, ZHANG L, JIANG D. Surface oxygen vacancies on Co3O4 mediated catalytic formaldehyde oxidation at room temperature[J]. Catal Sci Technol, 2016, 6:3845-3853. doi: 10.1039/C5CY01709B [15] HUANG Y C, FAN W J, LONG B, LI H B. Alkali-modified non-precious metal 3D-NiCo2O4 nanosheets for efficient formaldehyde oxidation at low temperature[J]. J Mater Chem A, 2016, 4(10):3648-3654. doi: 10.1039/C5TA09370H [16] 崔维怡, 惠继星, 谭乃迪.负载型铂催化剂催化氧化甲醛的研究进展[J].化工进展, 2017, 36(10):3711-3719. http://d.old.wanfangdata.com.cn/Periodical/hgjz201710025CUI Wei-yi, HUI Ji-xing, TAN Nai-di. Research progress on catalytic oxidation of formaldehyde over supported platinum catalysts[J]. Chem Ind Eng Prog, 2017, 36(10):3711-3719. http://d.old.wanfangdata.com.cn/Periodical/hgjz201710025 [17] GUO J H, LIN C X, JIANG C J, ZHANG P Y. Review on noble metal-based catalysts for formaldehyde oxidation at room temperature[J].Appl Surf Sci, 2019, 475:237-255. doi: 10.1016/j.apsusc.2018.12.238 [18] AN N H, YU Q S, LIU G, LI S Y, JIA M J, ZHANG W X. Complete oxidation of formaldehyde at ambient temperature over supported Pt/Fe2O3 catalysts prepared by colloid-deposition method[J]. J Hazard Mater, 2011, 186(10):1392-1397. http://www.sciencedirect.com/science/article/pii/S0304389410015980 [19] XU Z H, YU J G, JARONIEC M. Efficient catalytic removal of formaldehyde at room temperature using AlOOH nanoflakes with deposited Pt[J]. Appl Catal B:Environ, 2015, 163(2):306-312. http://d.old.wanfangdata.com.cn/Conference/9171952 [20] YAN Z X, XU Z H, YU J G, JARONIEC M. Highly active mesoporous ferrihydrite supported Pt catalyst for formaldehyde removal at room temperature[J]. Environ Sci Technol, 2015, 49(11):6637-6644. doi: 10.1021/acs.est.5b00532 [21] CUI W Y, YUAN X L, WU P, ZHENG B, ZHANG W X, JIA M J. Catalytic properties of Al2O3 supported Pt-FeOx catalysts for complete oxidation of formaldehyde at ambient temperature[J]. RSC Adv, 2015, 5(126):104330-104336. doi: 10.1039/C5RA19151C [22] 郑彬, 甘涛, 吴淑杰, 刘钢, 张文祥等. Pt-FeOx催化剂微结构对催化CO氧化性能的影响[J].无机化学学报, 2018, 34(6):1065-1070. http://d.old.wanfangdata.com.cn/Periodical/wjhxxb201806008ZHENG Bin, GAN Tao, WU Shu-jie, LIU Gang, ZHANG Wen-xiang. Influence of microstructure of Pt-FeOx catalyst on the catalytic CO oxidation[J]. Chin J Inorg Chem, 2018, 34(6):1065-1070. http://d.old.wanfangdata.com.cn/Periodical/wjhxxb201806008 [23] QI L F, CHENG B, YU J G, HO W K. High-surface area mesoporous Pt/TiO2 hollow chains for efficient formaldehyde decomposition at ambient temperature[J]. J Hazard Mater, 2016, 301:522-530. doi: 10.1016/j.jhazmat.2015.09.026 [24] CHEN G X, ZHAO Y, FU G, DUCHESNE P N, GU L, ZHENG Y P, WENG X F, CHEN M S, ZHANG P, PAO C W, LEE J F, ZHENG N F. Interfacial effects in iron-nickel hydroxide-platinum nanoparticles enhance catalytic oxidation[J]. Science, 2014, 344:495-499. doi: 10.1126/science.1252553 [25] XU L S, ZHANG W H, ZHANG Y L, WU Z F, CHEN B H, JIANG Z Q, MA Y S, YANG J L, HUANG W X. Oxygen vacancy-controlled reactivity of hydroxyls on an FeO(111) monolayer film[J]. J Phys Chem C, 2011, 115(14):6815-6824. doi: 10.1021/jp200423j [26] ZHENG B, LIU G, GENG L L, CUI J Y, WU S J, WU P, JIA M J, YAN W F, ZHANG W X. Role of the FeOx support in constructing high-performance Pt/FeOx catalysts for low-temperature CO oxidation[J]. Catal Sci Technol, 2016, 6(5):1546-1554. doi: 10.1039/C5CY00840A [27] CHEN B B, ZHU X B, CROCKER M, WANG Y, SHI C. FeOx-supported gold catalysts for catalytic removal of formaldehyde at room temperature[J]. Appl Catal B:Environ, 2014, 154-155:73-81. doi: 10.1016/j.apcatb.2014.02.009 [28] JIA J F, SHEN J Y, LIN L W, XU Z S, ZHANG T, LIANG D B. A study on reduction behaviors of the supported platinum-iron catalysts[J]. J Mol Catal A:Chem, 1999, 138:177-184. doi: 10.1016/S1381-1169(98)00147-2 [29] AN N H, DUCHESNE P N, LI S Y, WU P, ZHANG W L, LEE J F, CHENG S, ZHANG P, JIA M J, ZHANG W X. Size effects of platinum colloid particles on the structure and CO oxidation properties of supported Pt/Fe2O3 catalysts[J]. J Phys Chem C, 2013, 117:21254-21262. doi: 10.1021/jp404266p [30] AN N H, YUAN X L, PAN B, LI Q L, LI S Y, ZHANG W X. Design of a highly active Pt/Al2O3 catalyst for low-temperature CO oxidation[J]. RSC Adv, 2014, 4(72):38250-38257. doi: 10.1039/C4RA05646A -





 下载:
下载: