-
摘要: 采用水热合成法制备了无模板剂ZSM-5分子筛并用正硅酸甲酯(TMOS)对其进行外表面修饰改性,利用XRD、SEM、29Si MAS NMR、27Al MAS NMR、NH3-TPD、BET和UV-vis DRS对合成分子筛的物相、形貌和酸性等进行了表征,并将其应用于催化丁烯裂解反应。研究表明,经水热合成的无模板剂ZSM-5结晶度较好,与添加模板剂合成的ZSM-5拥有相似的孔道结构和晶体结构以及相近的酸量,但在酸中心分布上有明显差异:孔道内酸中心数量增加且分布更加均匀,孔道交叉处酸中心数量减少;经过外表面修饰改性后,ZSM-5分子筛外表面部分不具备择形性的酸中心被钝化,使其择形选择能力增强。在催化丁烯裂解反应中,用TMOS进行外表面修饰改性的无模板剂ZSM-5分子筛作为催化剂能够有效抑制副反应的发生,丙烯和乙烯的总收率高达58%。Abstract: The template-free ZSM-5 was prepared by hydrothermal synthesis and then modified by tetramethoxysilane (TMOS).The structure, morphology, and acidity of all samples were studied by various techniques, such as XRD, SEM, 29Si MAS NMR, 27Al MAS NMR, NH3-TPD, BET and UV-vis DRS.Comparing with the zeolites synthesized by traditional method, the template-free ZSM-5 exhibited the analogical acidity, morphology and structure, but obvious difference in acid distribution.Due to the absence of the structure directing agent, the template-free ZSM-5 possessed more acid sites situated at pore channels of catalyst and less acid sites stayed at the intersection of straight and sinusoidal channels.Consequently, the shape selectivity enhanced significantly.After TMOS modification, the non-shape acid sites located at the external surface were covered by a single SiO2 layer.The template-free ZSM-5 achieved the highest total yield of ethylene and propylene, longest working life-span and lowest level of coke deposition among the studied catalysts, ascribing to the suppression of the side reactions.
-
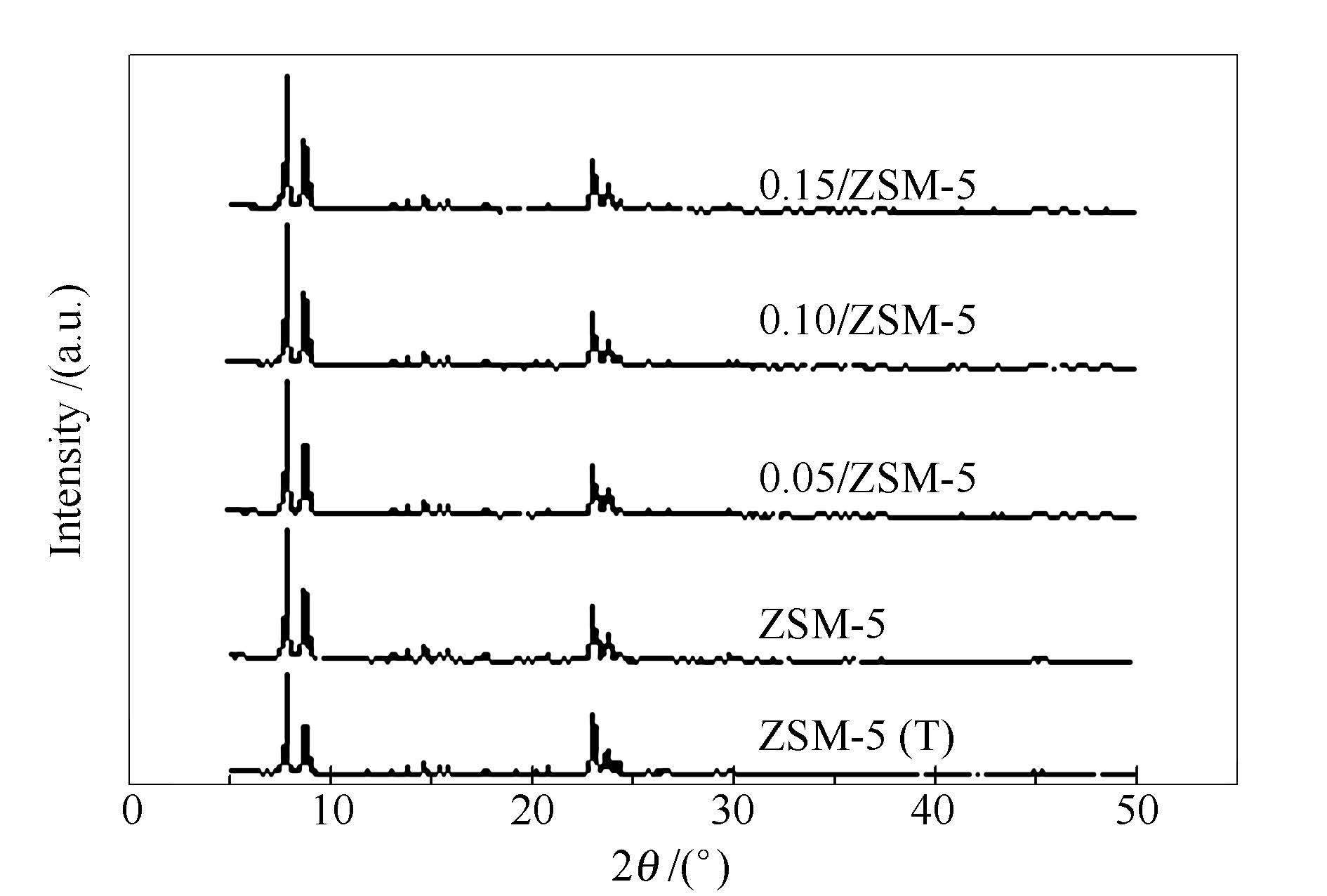
表 1 分子筛的相对结晶度
Table 1 Relative crystallinity of zeolites
Sample Relative crystallinity /% ZSM-5(T) 99.79 ZSM-5 98.59 0.05/ZSM-5 95.40 0.10/ZSM-5 96.51 0.15/ZSM-5 97.76 表 2 分子筛的物理性质
Table 2 Physical properties of zeolites
Sample Pore volume v/(cm3·g-1) Micropore volume v/(cm3·g-1) Surface area A/ (m2·g-1) ZSM-5(T) 0.192 0.058 307.6 ZSM-5 0.188 0.051 303.5 0.15/ZSM-5 0.187 0.054 297.6 surface area: BET surface area; micropore volume: t-plot method 表 3 分子筛的总酸量
Table 3 Acidic properties of zeolites
Sample Total acid sites/(mmol·g-1) ZSM-5(T) 0.433 2 ZSM-5 0.412 4 0.15/ZSM-5 0.319 8 -
[1] XUE N, CHEN X, NIE L, GUO X, DING W, CHEN Y, GU M, XIE Z. Understanding the enhancement of catalytic performance for olefin cracking:Hydrothermally stable acids in P/HZSM-5[J]. J Catal, 2007, 248(1):20-28. doi: 10.1016/j.jcat.2007.02.022 [2] LIU D, CHOI W C, LEE C W, KANG N Y, LEE Y J, SHIN C H, PARK Y K. Steaming and washing effect of P/HZSM-5 in catalytic cracking of naphtha[J]. Catal Today, 2011, 164(1):154-157. doi: 10.1016/j.cattod.2010.10.091 [3] TZOULAKI D, JENTYS A, PÉREZ-RAMÍREZ J, EGEBLAD K, LERCHER J A. On the location, strength and accessibility of Brönsted acid sites in hierarchical ZSM-5 particles[J]. Catal Today, 2012, 198(1):3-11. doi: 10.1016/j.cattod.2012.03.078 [4] ZHU X, LIU S, SONG Y, XU L. Catalytic cracking of C4 alkenes to propene and ethene:Influences of zeolites pore structures and Si/Al2 ratios[J]. Appl Catal A:Gen, 2005, 288(1/2):134-142. [5] XUE N, NIE L, FANG D, GUO X, SHEN J, DING W, CHEN Y. Synergistic effects of tungsten and phosphorus on catalytic cracking of butene to propene over HZSM-5[J]. Appl Catal A:Gen, 2009, 352(1/2):87-94. [6] ZENG P, LIANG Y, JI S, SHEN B, LIU H, WANG B, ZHAO H, LI M. Preparation of phosphorus-modified PITQ-13 catalysts and their performance in 1-butene catalytic cracking[J]. J Energy Chem, 2014, 23(2):193-200. doi: 10.1016/S2095-4956(14)60135-2 [7] CHEN C J, RANGARAJAN S, HILL I M, BHAN A. Kinetics and Thermochemistry of C4-C6 Olefin Cracking on HZSM-5[J]. ACS Catal, 2014, 4(7):2319-2327. doi: 10.1021/cs500119n [8] LI J, LI T, MA H, SUN Q, YING W, FANG D. Effect of impregnating Fe into P-modified HZSM-5 in the coupling cracking of butene and pentene[J]. Ind Eng Chem Res, 2015, 54(6):1796-17805. doi: 10.1021/ie504629p [9] LEE J, HONG U G, HWANG S, YOUN M H, SONG I K. Catalytic cracking of C5 raffinate to light olefins over lanthanum-containing phosphorous-modified porous ZSM-5:Effect of lanthanum content[J]. Fuel Process Technol, 2013, 109:189-195. doi: 10.1016/j.fuproc.2012.10.017 [10] LI J, MA H, SUN Q, YING W, FANG D. Effect of iron and phosphorus on HZSM-5 in catalytic cracking of 1-butene[J]. Fuel Process Technol, 2014, 134:32-38. [11] GAO X, TANG Z, LU G, CAO G, LI D, TAN Z. Butene catalytic cracking to ethylene and propylene on mesoporous ZSM-5 by desilication[J]. Solid State Sci, 2010, 12(7):1278-1282. doi: 10.1016/j.solidstatesciences.2010.04.020 [12] XU R F, LIU J X, LIANG C C, JIA W H, LI F F, GUO H C. Effect of alkali metal ion modification on the catalytic performance of nano-HZSM-5 zeolite in butene cracking[J]. J Fuel Chem Technol, 2011, 39(6):449-454. doi: 10.1016/S1872-5813(11)60029-7 [13] WAKUI K, SATOH K, SAWADA G, SHIOZAWA K, MATANO K, SUZUKI K, HAYAKAWA T, YOSHIMURA Y, MURATA K, MIZUKAMI F. Cracking of n, -butane over alkaline earth-containing HZSM-5 catalysts[J]. Catal Lett, 2002, 84(3/4):259-264. doi: 10.1023/A:1021448508130 [14] XIAONING W, ZHEN Z, CHUNMING X, AIJUN D, LI Z, GUIYUAN J. Effects of light rare earth on acidity and catalytic performance of HZSM-5 zeolite for catalytic cracking of butane to light olefins[J]. J Rare Earth, 2007, 25(3):321-328. doi: 10.1016/S1002-0721(07)60430-X [15] XUE N, LIU N, NIE L, YU Y, GU M, PENG L, GUO X, DING W. 1-Butene cracking to propene over P/HZSM-5:Effect of lanthanum[J]. J Mol Catal A:Chem, 2010, 327(1/2):12-19. [16] WEBER R W, M LLER K P, UNGER M, O'CONNOR C T. The chemical vapour and liquid deposition of tetraethoxysilane on the external surface of ZSM-5[J]. Microporous Mesoporous Mater, 1998, 23(3/4):179-187. [17] WEBER R W, M LLER K P, O'CONNOR C T. The chemical vapour and liquid deposition of tetraethoxysilane on ZSM-5, mordenite and beta[J]. Microporous Mesoporous Mater, 2000, 35-36(0):533-543. [18] ZHU Z, XIE Z, CHEN Q, KONG D, LI W, YANG W, LI C. Chemical liquid deposition with polysiloxane of ZSM-5 and its effect on acidity and catalytic properties[J]. Microporous Mesoporous Mater, 2007, 101(1/2):169-175. [19] 李淑娟, 袁桂梅, 薛扬, 吴韬, 陈胜利, 王桂敏. 硅源对 ZSM-5 分子筛合成和催化性能的影响[J]. 工业催化, 2014, 22(12):915-921. http://www.cnki.com.cn/Article/CJFDTOTAL-GYCH201412007.htmLI Shu-juan, YUAN Gui-mei, XUE Yang, WU Tao, CHEN Sheng-li, WANG Gui-min. Effects of silicon sources on synthesis and catalytic properties of ZSM-5 zeolites[J]. Ind Catal, 2014, 22(12):915-921. http://www.cnki.com.cn/Article/CJFDTOTAL-GYCH201412007.htm [20] 薛扬, 袁桂梅, 陈胜利, 李淑娟, 袁锐. ZSM-5 分子筛的磷改性及其碳四烯烃催化裂解性能[J]. 工业催化, 2014, 22(5):357-362. http://www.cnki.com.cn/Article/CJFDTOTAL-GYCH201405008.htmXUE Yang, YUAN Gui-mei, CHEN Sheng-li, LI Shu-juan, YUAN Rui. Phosphorus modified ZSM-5 molecular sieves and their catalytic performance for the cracking of butylenes[J]. Ind Catal, 2014, 22(5):357-362. http://www.cnki.com.cn/Article/CJFDTOTAL-GYCH201405008.htm [21] DING W, MEITZNER G D, IGLESIA E. The effects of silanation of external acid sites on the structure and catalytic behavior of Mo/H-ZSM5[J]. J Catal, 2002, 206(1):14-22. doi: 10.1006/jcat.2001.3457 [22] RURREN X, WENRQIN P, JIRHONG Y. Chemistry C Zeolites and Porous Materials[M]. Beijing:Science Press, 2004. [23] KLINOWSKI J. Solid-state NMR studies of molecular sieve catalysts[J]. Chem Rev, 1991, 91(7):1459-1479. doi: 10.1021/cr00007a010 [24] SAZAMA P, DĚDEČEK J, G BOV V, WICHTERLOV B, SPOTO G, BORDIGA S. Effect of aluminium distribution in the framework of ZSM-5 on hydrocarbon transformation. Cracking of 1-butene[J]. J Catal, 2008, 254(2):180-189. doi: 10.1016/j.jcat.2007.12.005 [25] DĚDEČEK J, KAUCK D, WICHTERLOV B. Al distribution in ZSM-5 zeolites:An experimental study[J]. Chem Commun, 2001, 11:970-971. [26] DĚDEČEK J, KAUCK D, WICHTERLOV B. Co2+ ion siting in pentasil-containing zeolites, part 3:Co2+ ion sites and their occupation in ZSM-5:A VIS diffuse reflectance spectroscopy study[J]. Microporous Mesoporous Mater, 2000, 35-36(0):483-494. [27] DEDECEK J, KAUCKY D, WICHTERLOVA B, GONSIOROVA O. Co2+ ions as probes of Al distribution in the framework of zeolites. ZSM-5 study[J]. Phys Chem Chem Phys, 2002, 4(21):5406-5413. doi: 10.1039/B203966B [28] DĚDEČEK J, ČAPEK L, KAUCK D, SOBALÍK Z, WICHTERLOV B. Siting and distribution of the Co ions in beta zeolite:A UV-Vis-NIR and FTIR Study[J]. J Catal, 2002, 211(1):198-207. doi: 10.1016/S0021-9517(02)93697-3 [29] YOKOI T, MOCHIZUKI H, NAMBA S, KONDO J N, TATSUMI T. Control of the Al distribution in the framework of ZSM-5 zeolite and its evaluation by solid-state NMR technique and catalytic properties[J]. J Phys Chem C, 2015, 119(27):15303-15315. doi: 10.1021/acs.jpcc.5b03289 [30] IWASE Y, SAKAMOTO Y, SHIGA A, MIYAJI A, MOTOKURA K, KOYAMA T R, BABA T. Shape-selective catalysis determined by the volume of a zeolite cavity and the reaction mechanism for propylene production by the conversion of butene using a proton-exchanged zeolite[J]. J Phys Chem C, 2012, 116(8):5182-5196. doi: 10.1021/jp212549j [31] KOYAMA T R, HAYASHI Y, HORIE H, KAWAUCHI S, MATSUMOTO A, IWASE Y, SAKAMOTO Y, MIYAJI A, MOTOKURA K, BABA T. Key role of the pore volume of zeolite for selective production of propylene from olefins[J]. Phys Chem Chem Phys, 2010, 12(11):2541-2554. doi: 10.1039/b921927g [32] SMIT B, MAESEN T L. Towards a molecular understanding of shape selectivity[J]. Nature, 2008, 451(7179):671-678. doi: 10.1038/nature06552 [33] INAGAKI S, SHINODA S, KANEKO Y, TAKECHI K, KOMATSU R, TSUBOI Y, YAMAZAKI H, KONDO J N, KUBOTA Y. Facile fabrication of ZSM-5 zeolite catalyst with High Durability to coke formation during catalytic cracking of paraffins[J]. ACS Catal, 2013, 3(1):74-78. doi: 10.1021/cs300426k [34] URATA K, FURUKAWA S, KOMATSU T. Location of coke on HZSM-5 zeolite formed in the cracking of n-hexane[J]. Appl Catal A:Gen, 2014, 475:335-340. doi: 10.1016/j.apcata.2014.01.050 -





 下载:
下载: