Effect of calcination temperature on the catalytic performance of the hydrotalcite derived Ce/Cu/Zn-Al catalysts for hydrogen production via methanol steam reforming
-
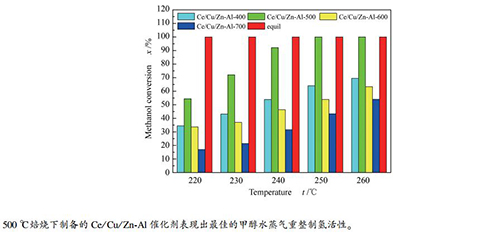
摘要: 采用原位合成法在γ-Al2O3载体表面上合成了Zn-Al水滑石,再采用顺序浸渍法制备了一系列Ce/Cu/Zn-Al催化剂,并采用XRD、BET、H2-TPR和XPS等手段对催化剂进行了表征,考察了焙烧温度对Ce/Cu/Zn-Al催化剂表面结构及其催化甲醇水蒸气重整制氢性能的影响。结果表明,焙烧温度主要影响了催化剂的Cu比表面积、表面氧空穴含量和Cu-Ce间相互作用。当焙烧温度为500℃时,催化剂Cu的比表面积较大,表面氧空穴含量较多,Cu-Ce间相互作用较强,因此,催化甲醇水蒸气重整制氢活性较好。当焙烧温度升高到700℃时,Cu物种主要以稳定的CuAl2O4尖晶石形式存在,不利于甲醇水蒸气重整制氢反应的进行,因此,催化活性较差。Abstract: ZnAl-LDHs was prepared by in-situ synthesis method on the surface of γ-Al2O3, and then a series Ce/Cu/Zn-Al catalysts were prepared by ordinal wet impregnation method. All the catalysts were characterized by XRD, BET, H2-TPR and XPS to investigate the effects of calcination temperature on the surface structure of Ce/Cu/Zn-Al catalyst and its catalytic performance in methanol steam reforming. The results showed that calcination temperature mainly influenced the specific surface area of copper, surface oxygen vacancy content and the interaction between Cu and Ce. When the calcination temperature is 500℃, the specific surface area of Cu is larger, the content of oxygen vacancy is higher and the interaction between Cu and Ce is stronger. Therefore, the catalytic activity of the catalysts for methanol steam reforming is the best. When the calcination temperature rises to 700℃, the Cu species mainly exist in the form of stable CuAl2O4 spinel, which is not conducive to the reaction of methanol steam reforming, resulting in lower catalytic activity.
-
Key words:
- calcination temperature /
- hydrotalcite /
- Cu-Al spinel /
- methanol steam reforming /
- hydrogen
-
表 1 催化剂的物化性质及其催化甲醇水蒸气重整反应中氢气产率
Table 1 Physical characteristics of the prepared catalysts and hydrogen production rate in methanol steam reforming
Catalyst ABET
/(m2·g-1)Pore volume
v/(cm3·g-1)Pore size
d/nmCu dispersiona/% Cu surface areaa
A/(m2·g-1)H2 production rateb
/(cm3·kg-1·s-1)Ce/Cu/Zn-Al-400 84.7 0.35 15.6 4.8 2.7 603.3 Ce/Cu/Zn-Al-500 109.6 0.41 15.3 11.5 \6.3 810.7 Ce/Cu/Zn-Al-600 77.2 0.38 18.8 3.7 2.0 505.4 Ce/Cu/Zn-Al-700 72.2 0.37 20.7 2.7 1.5 330.3 a: determined by N2O experiments;
b: reaction conditions: atmospheric, 240 ℃, W/M=1.2 : 1, GHSV=800 h-1, no carrier gas表 2 不同焙烧温度下制备催化剂的Ce 3d和O 1s XPS拟合结果
Table 2 Ce 3d and O 1s XPS curve-fitting results of catalysts calcined at various temperatures
Catalyst Ce3+ /(Ce3++ Ce4+)/% Oads/(Oads+O-OH+Olatt)w/% Ce/Cu/Zn-Al-400 21.77 0.62 Ce/Cu/Zn-Al-500 21.89 0.40 Ce/Cu/Zn-Al-600 21.61 0.39 Ce/Cu/Zn-Al-700 20.58 0.27 -
[1] RYAN J G, KHALID A A, WILLIAM H G. Thermochemical production of hydrogen from hydrogen sulfide with iodine thermochemical cycles[J]. Int J Hydrogen Energy, 2018, 43(29):12939-12947. doi: 10.1016/j.ijhydene.2018.04.217 [2] CLAUDE L. From hydrogen production by water electrolysis to its utilization in a PEM fuel cell or in a SO fuel cell:Some considerations on the energy efficiencies[J]. Int J Hydrogen Energy, 2016, 41(34):15415-15425. doi: 10.1016/j.ijhydene.2016.04.173 [3] HOSSAIN M A, JEWARATNAM J, GANESAN P. Prospect of hydrogen production from oil palm biomass by thermochemical processe-A review[J]. Int J Hydrogen Energy, 2016, 41(38):16637-16655. doi: 10.1016/j.ijhydene.2016.07.104 [4] SANDRA S, HUGO S, LUCIA B, SOUSA J M, MENDES A. Catalysts for methanol steam reforming-A review[J]. Appl Catal B:Environ, 2010, 99(1/2):43-57. http://www.sciencedirect.com/science/article/pii/S0926337310002584 [5] 苏石龙, 张磊, 张艳, 雷俊腾, 桂建州, 刘丹, 刘道胜, 潘立卫.千瓦级PEMFC甲醇水蒸气重整制氢过程热力学模拟[J].石油化工高等学校学报, 2015, 28(2):19-25. doi: 10.3969/j.issn.1006-396X.2015.02.004SU Shi-long, ZHANG Lei, ZHANG Yan, LEI Jun-teng, GUI Jian-zhou, LIU Dan, LIU Dao-sheng, PAN Li-wei. Thermodynamic Simulation for Hydrogen Production in the Methanol Steam Reforming System of Kilowatt PEMFC[J]. J Petrochem Univ, 2015, 28(2):19-25. doi: 10.3969/j.issn.1006-396X.2015.02.004 [6] SANCHES S G, FLORES J H, PAIS DA SILVA M I. Cu/ZnO and Cu/ZnO/ZrO2 catalysts used for methanol steam reforming[J]. Mol Catal, 2018, 454:55-62. doi: 10.1016/j.mcat.2018.05.012 [7] XU T K, ZOU J, TAO W T, ZHANG S Y, CUI L, ZENG F L, WANG D Z, CAI W J. Co-nanocasting synthesis of Cu based composite oxide and itspromoted catalytic activity for methanol steam reforming[J]. Fuel, 2018, 183:238-244. http://www.sciencedirect.com/science/article/pii/S0016236116305403 [8] LI J, ZHANG Q J, LONG X, QI P, LIU Z T, LIU Z W. Hydrogen production for fuel cells via steam reforming of dimethyl ether over commercial Cu/ZnO/Al2O3 and zeolite[J]. Chem Eng J, 2012, 187:299-305. doi: 10.1016/j.cej.2012.01.126 [9] CHOI Y, FUTAGAMI K, FUTAGAMI K, FUJITANI T, NAKAMURA J. The role of ZnO in Cu/ZnO methanol synthesis catalysts-morphology effect or active site model[J]. Appl Catal A:Gen, 2001, 208(1/2):163-167. http://www.sciencedirect.com/science/article/pii/S0926860X00007122 [10] XIAO S, ZHANG Y F, GAO P, ZHONG L S, LI X P, ZHANG Z Z, WANG H, WEI W, SUN Y H. Highly efficient Cu-based catalysts via hydrotalcite-like precursors for CO2 hydrogenation to methanol[J]. Catal Today, 2017, 281:327-336. doi: 10.1016/j.cattod.2016.02.004 [11] HAMMOUD D, GENNEQUIN C, ABOUKAIS A, AAD E A. Steam reforming of methanol over x% Cu/Zn-Al 400500 based catalysts for production of hydrogen:Preparation by adopting memory effect of hydrotalcite and behavior evaluation[J]. Int J Hydrogen Energy, 2015, 40(2):1283-1297. doi: 10.1016/j.ijhydene.2014.09.080 [12] HE J P, YANG Z X, ZHANG L, LI Y, PAN L W. Cu supported on ZnAl-LDHs precursor prepared by in-situ synthesis method on γ-Al2O3 as catalytic material with high catalytic activity for methanol steam reforming[J]. Int J Hydrogen Energy, 2017, 42(15):9930-9937. doi: 10.1016/j.ijhydene.2017.01.229 [13] 贺建平, 张磊, 陈琳, 杨占旭, 佟宇飞. CeO2改性Cu/Zn-Al水滑石衍生催化剂对甲醇水蒸气重整制氢性能的影响[J].高等学校化学学报, 2017, 38:1822-1828. doi: 10.7503/cjcu20170158HE Jian-ping, ZHANG Lei, CHEN Lin, YANG Zhan-xu, TONG Yu-fei. Effect of CeO2 on Cu/Zn-Al catalysts derived from hydrotalcite precursor for methanol steam reforming[J]. Chem J Chin Univ, 2017, 38:1822-1828. doi: 10.7503/cjcu20170158 [14] 杨淑倩, 贺建平, 张娜, 隋晓伟, 张磊, 杨占旭.稀土掺杂改性对Cu/ZnAl水滑石衍生催化剂甲醇水蒸气重整制氢性能的影响[J].燃料化学学报, 2018, 46(2):179-188. doi: 10.3969/j.issn.0253-2409.2018.02.007YANG Shu-qian, HE Jian-ping, ZHANG Na, SUI Xiao-wei, ZHANG Lei, YANG Zhan-xu. Effect of rare-earth element modification on the performance of Cu/ZnAl catalysts derived from hydrotalcite precursor in methanol steam reforming[J]. J Fuel Chem Technol, 2018, 46(2):179-188. doi: 10.3969/j.issn.0253-2409.2018.02.007 [15] 杨淑倩, 张娜, 贺建平, 张磊, 王宏浩, 白金, 张健, 刘道胜, 杨占旭. Ce的浸渍顺序对Cu/Zn-Al水滑石衍生催化剂用于甲醇水蒸气重整制氢性能的影响[J].燃料化学学报, 2018, 46(4):479-488. doi: 10.3969/j.issn.0253-2409.2018.04.014YANG Shu-qian, ZHANG Na, HE Jian-ping, ZHANG Lei, WANG Hong-hao, BAI Jin, ZHANG Jian, LIU Dao-sheng, YANG Zhan-xu. Effect of impregnation sequence of Ce on the performance of Cu/Zn-Al catalysts derived from hydrotalcite precursor in methanol steam reforming[J]. J Fuel Chem Technol, 2018, 46(4):479-488. doi: 10.3969/j.issn.0253-2409.2018.04.014 [16] 刘玉娟, 许骥, 佟宇飞, 张娜, 张磊, 刘道胜, 韩蛟, 张财顺.氧化铈纳米材料合成方法的研究进展[J].辽宁石油化工大学学报, 2017, 37(5):8-12. doi: 10.3969/j.issn.1672-6952.2017.05.002LIU Yu-Juan, XU Ji, TONG Yu-fei, ZHANG Na, ZHANG Lei, LIU Dao-sheng, HAN Jiao, ZHANG Cai-shun. Progress in research of the synthesis methods of nanometer ceria[J]. J Liaoning Univ Pet Chem Technol, 2017, 37(5):8-12. doi: 10.3969/j.issn.1672-6952.2017.05.002 [17] 张秋林, 徐海迪, 李伟, 林涛, 龚茂初, 陈耀强.焙烧温度对MnO2-CeO2/Zr0.25Ti0.25Al0.5O1.75整体式催化剂NH3低温选择性催化还原NO性能的影响[J].催化学报, 2010, 31(2):229-235. http://d.wanfangdata.com.cn/Periodical/cuihuaxb201002017ZHANG Qiu-lin, XU Hai-di, LI Wei, LIN Tao, GONG Mao-chun, CHEN Yao-qiang. Influence of calcination temperature on performance of monolith catalyst MnO2-CeO2/Zr0.25Ti0.25Al0.5O1.75 for selective catalytic reduction of NO by NH3 at low temperature[J]. Chin J Catal, 2010, 31(2):229-235. http://d.wanfangdata.com.cn/Periodical/cuihuaxb201002017 [18] BIALAS A, KUSTROWSKI P, DUDEK B, PIWOWARSKA Z, WACH A, MICHALIK M, KOZAK M. Copper-aluminum oxide catalysts for total oxidation of toluene synthesized by thermal decomposition of co-precipitated precursors[J]. Thermochim Acta, 2014, 590:191-197. doi: 10.1016/j.tca.2014.06.027 [19] 方书农, 姜明, 伏义路, 林培琰, 乔山, 谢亚宁.不同焙烧温度对Cu/γ-Al2O3催化剂铜物种结构的影响[J].物理化学学报, 1994, 10(7):623-627. doi: 10.3866/PKU.WHXB19940709FANG Shu-nong, JIANG Ming, FU Yi-lu, LIN Pei-yan, QIAO Shan, XIE Ya-ning. The effect of different calcination temperature on the structure of Cu/γ-Al2O3 catalysts[J]. Acta Phys Chim Sin, 1994, 10(7):623-627. doi: 10.3866/PKU.WHXB19940709 [20] 孙蛟, 任国卿, 黄玉辉, 陈晓蓉, 梅华.焙烧温度对CuMgAl催化剂催化糠醛气相加氢制糠醇性能的影响[J].燃料化学学报, 2017, 45(1):43-47. doi: 10.3969/j.issn.0253-2409.2017.01.007SUN Jiao, REN Guo-qing, HUANG Yu-hui, CHEN Xiao-rong, MEI Hua. Effect of calcination temperature on the catalytic performance of CuMgAl catalysts for furfural gas phase selective hydrogenation to furfuryl alcohol[J]. J Fuel Chem Technol, 2017, 45(1):43-47. doi: 10.3969/j.issn.0253-2409.2017.01.007 [21] BASAG S, PIWOWARSKA Z, KOWALCZYK A, WEGRZYN A, BARAN R, GIL B, MICHALIK M, CHMIELARZ L. Cu-Mg-Al hydrotalcite-like materials as precursors of effective catalysts for selective oxidation of ammonia to dinitrogen-The influence of Mg/Al ratio and calcination temperature[J]. Appl Clay Sci, 2016, 129:122-130. doi: 10.1016/j.clay.2016.05.019 [22] ZHANG L, PAN L W, NI C J, SUN T J, ZHAO S S, WANG S D, WANG A J, HU Y K. CeO2-ZrO2-promoted CuO/ZnO catalyst for methanol steam reforming[J]. Int J Hydrogen Energy, 2013, 38(11):4397-4406. doi: 10.1016/j.ijhydene.2013.01.053 [23] GUO X M, MAO D S, LU G Z, WANG S, WU G S. CO2 hydrogenation to methanol over Cu/ZnO/ZrO2 catalysts prepared via a route of solid-state reaction[J].Catal Commun, 2011, 12(12):1095-1098. doi: 10.1016/j.catcom.2011.03.033 [24] SHIM J O, NA H S, JHA A, JANG W J, JEONG D W, NAH I W, JEON B H, ROH H S. Effect of preparation method on the oxygen vacancy concentration of CeO2-promoted Cu/γ-Al2O3 catalysts for HTS reactions[J]. Chem Eng J, 2016, 306:908-915. doi: 10.1016/j.cej.2016.08.030 [25] BYOUNG K K, DAE S P, YANG S Y, JONGHEOP Y. Preparation and characterization of nanocrystalline CuAl2O4 spinel catalysts by sol-gel method for the hydrogenolysis of glycerol[J]. Catal Commun, 2012, 24:90-95. doi: 10.1016/j.catcom.2012.03.029 [26] 覃发玠, 刘雅杰, 庆绍军, 侯晓宁, 高志贤.甲醇制氢铜铝尖晶石缓释催化剂的研究-不同铜源合成的影响[J].燃料化学学报, 2017, 45(12):1481-1488. doi: 10.3969/j.issn.0253-2409.2017.12.010QIN Fa-jie, LIU Ya-jie, QING Shao-jun, HOU Xiao-ning, GAO Zhi-xian. Cu-Al spinel as a sustained release catalyst for H2 production from methanol steam reforming:Effects of different copper sources[J]. J Fuel Chem Technol, 2017, 45(12):1481-1488. doi: 10.3969/j.issn.0253-2409.2017.12.010 [27] WANG J, ZHONG L P, LU J C, CHEN R, LEI Y Q, CHEN K Z, HAN C H, HE S F, WAN G P, LUO Y M. A solvent-free method to rapidly synthesize CuO-CeO2 catalysts to enhance their CO preferential oxidation:Effects of Cu loading and calcination temperature[J]. Mol Catal, 2017, 443:241-252. doi: 10.1016/j.mcat.2017.10.012 [28] LUO M F, FANG P, HE M, XIE Y L. In situ XRD, Raman, and TPR studies of CuO/Al2O3 catalysts for CO oxidation[J]. J Mol Catal A:Chem, 2005, 239(1/2):243-248. http://www.sciencedirect.com/science/article/pii/S1381116905004164 [29] 张磊, 雷俊腾, 田园, 胡鑫, 白金, 刘丹, 杨义, 潘立卫.前驱体和沉淀剂浓度对CuO/ZnO/CeO2-ZrO2甲醇水蒸气重整制氢催化剂性能的影响[J].燃料化学学报, 2015, 43(11):1366-1374. doi: 10.3969/j.issn.0253-2409.2015.11.012ZHANG Lei, LEI Jun-teng, TIAN Yuan, HU Xin, BAI Jin, LIU Dan, YANG Yi, PAN Li-wei. Effect of precursor and precipitant concentration on the performance of CuO/ZnO/CeO2-ZrO2 catalyst for methanol steam reforming[J]. J Fuel Chem Technol, 2015, 43(11):1366-1374. doi: 10.3969/j.issn.0253-2409.2015.11.012 [30] TANG D M, LIU G, LI F, TAN J, LIU C, LU G Q, CHENG H M. Synthesis and photoelectrochemical property of Urchin-like Zn/ZnO core-shell structures[J]. J Phys Chem C, 2009, 113(25):11035-11040. doi: 10.1021/jp8107254 [31] SEO Y S, CHOI T Y, HA J, JEONG D Y, LEE S Y, KIM D. Enhancement of stability of aqueous suspension of alumina nanoparticles by femtosecond laser irradiation[J]. J Appl Phys, 2015, 118:114906. doi: 10.1063/1.4931373 [32] WANG C, CHENG Q P, WANG X L, MA K, BAI X Q, TAN S R, TIAN Y, TONG D, ZHENG L R, ZHANG J, LI X G. Enhanced catalytic performance for CO preferential oxidation over CuO catalysts supported on highly defective CeO2 nanocrystals[J]. Appl Surf Sci, 2017, 422:932-943. doi: 10.1016/j.apsusc.2017.06.017 [33] 张国强, 郭天玉, 郑华艳, 李忠.焙烧温度对CuCe/Ac催化剂甲醇氧化羰基化性能的影响[J].燃料化学学报, 2016, 44(6):674-679. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18844.shtmlZHANG Guo-qiang, GUO Tian-yu, LI Zhong. Effect of calcination temperature on catalytic performance of CuCe/Ac catalysts for oxidative carbonylation of methanol[J]. J Fuel Chem Technol, 2016, 44(6):674-679. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18844.shtml [34] FAN J, WU X D, WU X D, LIANG Q, RAN R, WENG D. Thermal ageing of Pt on low-surface-area CeO2-ZrO2-La2O3 mixed oxides:Effect on the OSC performance[J]. Appl Catal B:Environ, 2008, 81(1/2):38-48. http://www.sciencedirect.com/science/article/pii/S0926337307004183 [35] LIOTTA L F, CARLO G D, PANTALEO G, VENEZIA A M, DEGANELLO G. Co3O4/CeO2 composite oxides for methane emissions abatement:Relationship between Co3O4-CeO2 interaction and catalytic activity[J]. Appl Catal B:Environ, 2006, 66(3/4):217-227. [36] LIANG F L, YU Y, ZHOU W, XU X Y, ZHU Z H. Highly defective CeO2 as a promoter for efficient and stable water oxidation[J]. J Mater Chem A, 2015, 3(2):634-640. doi: 10.1039/C4TA05770H [37] LIN S S, CHEN C L, CHANG D J, CHEN C C. Catalytic wet air oxidation of phenol by various CeO2 catalysts[J]. Water Res, 2002, 36(12):3009-3014. doi: 10.1016/S0043-1354(01)00539-5 -





 下载:
下载: