Effects of ZrO2 and Al2O3 on the performance of Mo-based catalysts in methanation
-
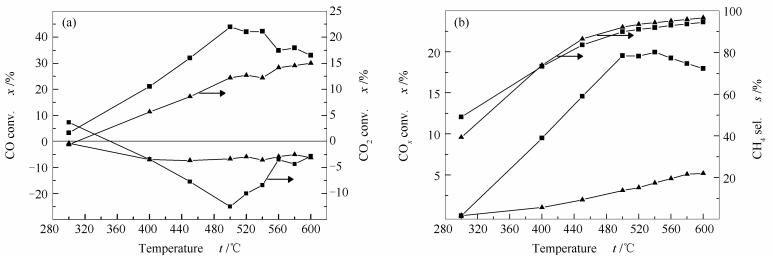
摘要: 通过共沉淀法制备了ZrO2和Al2O3载体,采用等体积浸渍法制备了MoO3质量分数为5%的Mo/ZrO2和Mo/Al2O3催化剂,并用于甲烷化反应。在三种反应气氛下对两种预硫化的Mo基催化剂进行评价,发现ZrO2载体均可显著促进甲烷化反应,同时能够促进水汽变换(WGS)反应。通过XRD、H2-TPR、XPS和TEM等表征发现,两种载体上Mo物种的硫化程度以及暴露的活性位数量不同,从而导致两种催化剂上催化性能差异显著。与Mo/Al2O3相比,Mo/ZrO2催化剂上的MoO3更易被还原,硫化程度也更高,并且Mo4+的含量更高,Mo6+的含量更低。虽然ZrO2载体上MoS2尺寸较大,边位置的Mo比例有所降低,但是由于MoS2沿ZrO2颗粒表面弯曲生长,使得MoS2基面成为反应的活性位;因此,Mo/ZrO2催化剂在甲烷化与WGS反应中表现出更优异的催化性能。Abstract: Mo/ZrO2 and Mo/Al2O3 catalysts with a MoO3 loading of 5% were prepared by incipient wetness impregnation method; the effect of support on the performance of Mo-based catalysts in methanation was then investigated in three different feeds. The results indicate that ZrO2 as a support can promote the methanation and water-gas shift (WGS) reaction. ZrO2 is beneficial to the sulfuration and reduction of MoO3. The Mo sulfidation degree and the content of Mo4+ on Mo/ZrO2 are higher than that on Mo/Al2O3. Besides, the curved MoS2 basal plane on Mo/ZrO2 can provide the active sites for methanation and WGS, which is effective to enhance the performance of Mo-based catalysts in methanation.
-
Key words:
- CO/CO2 hydrogenation /
- methanation /
- oxide supports /
- sulfidation degree
-
表 1 Mo/Al2O3和Mo/ZrO2催化剂在三种反应气氛下的CH4收率
Table 1 CH4 yields for methanation over the Mo/Al2O3 and Mo/ZrO2 catalysts in three different feed gases

表 2 反应前后催化剂表面Mo与S物种组成
Table 2 Composition of Mo and S species for the Mo/Al2O3 and Mo/ZrO2 catalysts

-
[1] LAI W, SONG W, PANG L, WU Z, ZHENG N, LI J, ZHENG J, YI X, FANG W. The effect of starch addition on combustion synthesis of NiMo-Al2O3 catalysts for hydrode sulfurization[J]. J Catal, 2013, 303:80-91. doi: 10.1016/j.jcat.2013.03.001 [2] HENSLEY J E, PYLYPENKO S, RUDDY D A. Deactivation and stability of K-CoMoSx mixed alcohol synthesis catalysts[J]. J Catal, 2014, 309:199-208. doi: 10.1016/j.jcat.2013.10.001 [3] SANTOS V P, LINDEN B, CHOJECKI A, BUDRONI G, CORTHALS S, SHIBATA H, MEIMA G R, KAPTEIJN F, MAKKEE M, GASCON J. Mechanistic insight into the synthesis of higher alcohols from syngas:The role of K promotion on MoS2 catalysts[J]. ACS Catal, 2013, 3(7):1634-1637. doi: 10.1021/cs4003518 [4] SASAKI T, SUZUKI T, TAKAOKA M. Reaction selectivity to hydrocarbons and solid-state carbon over molybdenum sulfide-based shift catalyst[J]. Appl Catal A:Gen, 2016, 514:83-90. doi: 10.1016/j.apcata.2015.11.049 [5] SINGH R, KUNZRU D, SIVAKUMAR S. Co-promoted MoO3 nanoclusters for hydrode sulfurization[J]. Catal Sci Technol, 2016, 6(15):5949-5960. doi: 10.1039/C5CY02221E [6] WANG B, DING G, SHANG Y, LV J, WANG H, WANG E, LI Z, MA X, QIN S, SUN Q. Effects of MoO3 loading and calcination temperature on the activity of the sulphur-resistant methanation catalyst MoO3/γ-Al2O3[J]. Appl Catal A:Gen, 2012, 431/432:144-150. doi: 10.1016/j.apcata.2012.04.029 [7] LI Z, TIAN Y, HE J, WANG B, MA X. High CO methanation activity on zirconia-supported molybdenum sulfide catalyst[J]. J Energy Chem, 2014, 23(5):625-632. doi: 10.1016/S2095-4956(14)60193-5 [8] GAO J J, LIU Q, GU F N, LIU B, ZHONG Z Y, SU F B. Recent advances in methanation catalysts for the production of synthetic natural gas[J]. Rsc Adv, 2015, 5(29):22759-22776. doi: 10.1039/C4RA16114A [9] WANG Z Z, HAN W F, LIU H Z. Hydrothermal synthesis of sulfur-resistant MoS2 catalyst for methanation reaction[J]. Catal Commun, 2016, 84:120-123. doi: 10.1016/j.catcom.2016.06.016 [10] 刘震, 王保伟, 王玮涵, 孟大钧, 李振花, 马新宾. B2O3负载量对MoO3/CeO2-Al2O3耐硫甲烷化性能的影响[J].化工学报, 2016, 67(9):3672-3677. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=hgsz201609019&dbname=CJFD&dbcode=CJFQLIU Zhen, WANG Bao-wei, WANG Wei-han, MENG Da-jun, LI Zhen-hua, MA Xin-bin, Impact of B2O3 loading on sulfur-resistant methanation activity of MoO3/CeO2-Al2O3 catalyst[J]. CIESC J, 2016, 67(9):3672-3677. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=hgsz201609019&dbname=CJFD&dbcode=CJFQ [11] 李振花, 曲江磊, 王玮涵, 王保伟, 马新宾.临CO2气氛下钼基催化剂耐硫甲烷化性能研究[J].燃料化学学报, 2016, 44(8):985-992. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18884.shtmlLI Zhen-hua, QU Jiang-lei, WANG Wei-han, WANG Bao-wei, MA Xin-bin. Effect of CO2 in syngas on methanation performance of Mo-based catalyst[J]. J Fuel Chem Technol, 2016, 44(8):985-992. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18884.shtml [12] DA SILVA D, LETICHEVSKY S, BORGES L, APPEL L. The Ni/ZrO2 catalyst and the methanation of CO and CO2[J]. Int J Hydrogen Energy, 2012, 37:8923-8928. doi: 10.1016/j.ijhydene.2012.03.020 [13] SHARMA S, KUMAR K, CHANDNANI Y, KUMAR V, GANGWAR B, SINGHAL A, DESHPANDE P. Mechanistic insights into CO2 methanation over Ru-substituted CeO2[J]. J Phys Chem C, 2016, 120(26):14101-14112. doi: 10.1021/acs.jpcc.6b03224 [14] 秦绍东, 龙俊英, 田大勇, 汪国高, 杨霞, 孙守理, 孙琦.不同载体负载的Mo基甲烷化催化剂[J].工业催化, 2014, 22(10):770-774. doi: 10.3969/j.issn.1008-1143.2014.10.008QIN Shao-dong, LONG Jun-ying, TIAN Da-yong, WANG Guo-gao, YANG Xia, SUN Shou-li, SUN Qi. Supported Mo-based catalysts with different carriers for methanation[J]. Ind Catal, 2014, 22(10):770-774. doi: 10.3969/j.issn.1008-1143.2014.10.008 [15] KIM M Y, HA S B, KOH D J, BYUN C, PARK E D. CO methanation over supported Mo catalysts in the presence of H2S[J]. Catal Commun, 2013, 35:68-71. doi: 10.1016/j.catcom.2013.02.004 [16] RAZZAQ R, LI C, USMAN M, SUZUKI K, ZHANG S. A highly active and stable Co4N/gamma-Al2O3 catalyst for CO and CO2 methanation to produce synthetic natural gas (SNG)[J]. Chem Eng J, 2015, 262:1090-1098. doi: 10.1016/j.cej.2014.10.073 [17] RAZZAQ R, ZHU H W, JIANG L, MUHAMMAD U, LI C S, ZHANG S J. Catalytic methanation of CO and CO2 in coke oven gas over Ni-Co/ZrO2-CeO2[J]. Ind Eng Chem Res, 2013, 52:2247-2256. doi: 10.1021/ie301399z [18] LIU J, WANG E, LV J, LI Z, WANG B, MA X, QIN S, SUN Q. Investigation of sulfur-resistant, highly active unsupported MoS2 catalysts for synthetic natural gas production from CO methanation[J]. Fuel Process Technol, 2013, 110:249-257. doi: 10.1016/j.fuproc.2013.01.003 [19] KATTEL S, YU W, YANG X, YAN B, HUANG Y, WAN W, LIU P, CHEN J G. CO2 hydrogenation over oxide-supported PtCo catalysts:The role of the oxide support in determining the product selectivity[J]. Angew Chem Int Ed, 2016, 55:7968-7973. doi: 10.1002/anie.201601661 [20] El-SHARKAWY E A, KHDER A S, AHMED A I. Structural characterization and catalytic activity of molybdenum oxide supported zirconia catalysts[J]. Microporous Mesoporous Mater, 2007, 102(1/3):128-137. http://www.sciencedirect.com/science/article/pii/S1387181106005750 [21] CUI F, LI G, LI X, LUA M, LI M. Enhancement of hydrodesulfurization of 4, 6-dimethyldibenzothiophene catalyzed by CoMo catalysts supported on carbon-covered gamma-Al2O3[J]. Catal Sci Technol, 2015, 5(1):549-555. doi: 10.1039/C4CY00814F [22] DINTER N, RUSANEN M, RAYBAUD P, KASZTELAN S, SILVA P, TOULHOAT H. Temperature-programmed reduction of unpromoted MoS2-based hydrodesulfurization catalysts:First-principles kinetic monte carlo simulations and comparison with experiments[J]. J Catal, 2010, 275:117-128. doi: 10.1016/j.jcat.2010.07.020 [23] AFANASIEV P. Calculation of MoS2 slabs morphology descriptors from transmission electron microscopy data revisited. Case study of the influence of citric acid and treatment conditions on the properties of MoS2/Al2O3[J]. Appl Catal A:Gen, 2017, 529:10-19. doi: 10.1016/j.apcata.2016.10.008 [24] LI H F, LI M F, CHU Y, LIU F, NIE H. Effect of different preparation methods of MoO3/Al2O3 catalysts on the existing states of Mo species and hydrode sulfurization activity[J]. Fuel, 2014, 116:168-174. doi: 10.1016/j.fuel.2013.07.127 [25] QIU L M, XU G T. Peak overlaps and corresponding solutions in the X-ray photoelectron spectroscopic study of hydrodesulfurization catalysts[J]. Appl Surf Sci, 2010, 256:3413-3417. doi: 10.1016/j.apsusc.2009.12.043 [26] LI H F, LI M F, NIE H. Tailoring the surface characteristic of alumina for preparation of highly active NiMo/Al2O3 hydrodesulfurization catalyst[J]. Microporous Mesoporous Mater, 2014, 188:30-36. doi: 10.1016/j.micromeso.2014.01.003 [27] NIKULSHIN P A, ISHUTENKO D I, MOZHAEV A A, MASLAKOV K I, PIMERZIN A A. Effects of composition and morphology of active phase of CoMo/Al2O3 catalysts prepared using Co2Mo10-heteropolyacid and chelating agents on their catalytic properties in HDS and HYD reactions[J]. J Catal, 2014, 312:152-169. doi: 10.1016/j.jcat.2014.01.014 [28] GAO D, DUAN A, ZHANG X, ZHAO Z, HONG E, LI J, WANG H. Synthesis of NiMo catalysts supported on mesoporous Al-SBA-15 with different morphologies and their catalytic performance of DBT HDS[J]. Appl Catal B:Environ, 2015, 165:269-284. doi: 10.1016/j.apcatb.2014.10.034 [29] HU K H, HU X G, XU Y F, PAN X Z. The effect of morphology and size on the photocatalytic properties of MoS2[J]. React Kinet Mech Catal, 2010, 100(1):153-163. doi: 10.1007/s11144-010-0173-3 -





 下载:
下载: