Structure and pyrolysis characteristics of enzymatic/mild acidolysis lignin isolated from palm kernel shell
-
摘要: 采用酶解/温和酸解法提取了棕榈壳和麦秆的木质素(EMALs),利用傅里叶红外光谱(FT-IR)、裂解器-气相色谱质谱联用(Py-GC/MS)和热重-红外联用(TG-FTIR)技术,对两种EMALs的化学结构和热解特性进行了对比研究,并采用Ozawa-Flynn-Wall方法计算了其热解反应的活化能。结果表明,棕榈壳EMAL和麦秆EMAL均为HGS型木质素。500℃下,两种EMALs的热解产物主要包括酚类、酸类和少量的醇类、醛酮类等化合物;棕榈壳EMAL热解酚类产物中H、G、S型单体酚类的比例分别为47.61%、25.64%和17.18%,而麦秆EMAL分别为23.66%、51.90%和15.50%。在热解反应主失重区(200-380℃),棕榈壳EMAL的主失重速率(50.80%/min)低于麦秆EMAL(78.63%/min);但棕榈壳EMAL热解同时存在肩状失重峰(265℃,27.40%/min),这与其较多H结构产物的释放相关。H型结构产物释放的放热效应降低了棕榈壳EMAL热解初期的活化能(20%,127.92 kJ/mol),同时使其热解过程(20%-80%)的平均活化能(152.32 kJ/mol)低于麦秆EMAL(161.75 kJ/mol)。Abstract: Firstly, lignin of palm kernel shell (PKS) and wheat straw (WS) were isolated by enzymatic/mild acidolysis method (EMAL). Then the functional groups and thermal decomposition characteristics of the two EMALs were analyzed with Fourier transform infrared spectroscopy (FT-IR), pyrolysis-gas chromatography/mass spectrometry (Py-GC/MS) and TG-FTIR. At last, the Ozawa-Flynn-Wall method was used to calculate the activation energy of pyrolysis of the two EMALs. FT-IR result show that both PKS-EMAL and WS-EMAL are Type HGS. Phenols, acids, few of alcohols, aldehydes and ketones are detected in volatile products from 500℃ pyrolysis of the two EMALs. Meanwhile, H-type, G-type and S-type phenols with proportions of 47.61%, 25.64% and 17.18% are obtained in phenolic products from PKS-EMAL pyrolysis; while they are 23.66%, 51.90% and 15.50%, respectively, in phenolic products of WS-EMAL. During 200-380℃, the main weight loss rate of PKS-EMAL pyrolysis is 50.80%/min, which is obviously lower than that (78.63%/min) of WS-EMAL. A shoulder peak of 27.40%/min at 265℃ is also observed in PKS-EMAL pyrolysis, which is closely related to release of H-derivatives during PKS-EMAL pyrolysis. The activation energy (127.92 kJ/mol) of PKS-EMAL pyrolysis at a conversion of 20% reduced by the exothermic effect corresponding torelease of H-derivatives, which was the main reason that the average activation energy (152.32 kJ/mol) of PKS-EMAL pyrolysis (20%-80%) was lower than that (161.75 kJ/mol) for WS-EMAL pyrolysis.
-
Key words:
- palm kernel shell /
- lignin /
- chemical structure /
- pyrolysis /
- activation energy
-
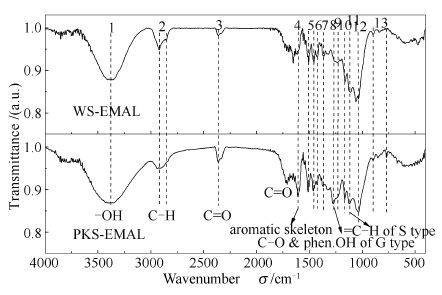
表 1 PKS-EMAL、WS-EMAL FT-IR谱图的解析[3, 10]
Table 1 FT-IR spectra analysis of EMALs isolated from PKS and WS
Num. in Figure 1 Wavenumber σ/cm-1 Band assignment PKS-EMAL WS-EMAL 1 3 403 3 403 O-H stretching 2 2 934 2 923, 2 855 C-H stretching 3 - 1 652 C=O stretching in conjugated aryl ketones 4 1 597 1 601 aromatic skeleton vibrations 5 1 465 1 462 C-H deformations 6 1 427 1 427 aromatic skeleton vibrations & C-H in plane deformations 7 1 367 1 367 aliphatic C-H stretching in-CH3 and phen.OH 8 1 271 1 269 C-O stretching in-OCH3 and phen.OH of G type 9 1 229 1 229 C-C+C-O+C=O stretching 10 1 160 1 160 C=O in ester groups, typical for HGS lignins 11 1 115 1 116 C-H stretching of typical S unit 12 1 036 1 036 aromatic C-H in plane deformation & C-O stretching 13 700-900 700-900 Aromatic hydrogen 表 2 PKS-EMAL、WS-EMAL 500 ℃热解生物油的主要组分及相对含量
Table 2 Main chemical constituents of bio-oils from pyrolysis of PKS-EMAL and WS-EMAL at 500 ℃
Retention time t/min Components of bio-oil Structuralformula Molecular formula Relative content w/% PKS-EMAL WS-EMAL Acids & alcohols 2.06 acetic acid 
C2H4O2 1.54 - 14.22 1, 6-anhydro-β-glucopyranose C6H10O5 - 2.17 16.61 tetradecanoic acid C14H28O2 1.23 1.62 18.36 cis-9-hexadecenoic acid C16H30O2 0.86 0.56 18.62 n-hexadecanoic acid C16H32O2 4.69 5.54 20.17 6-octadecenoic acid C18H34O2 1.28 2.88 20.42 octadecanoic acid C18H36O2 4.46 5.00 22.04 eicosanoic acid C20H40O2 0.34 0.56 22.73 dehydroabietic acid C20H28O2 - 2.13 23.45 phthalicacid, 2-ethylhexylester C16H22O4 3.25 3.99 total 17.65 24.45 Aldehydes & ketones 3.38 furfural 
C5H4O2 2.23 - 11.08 (Z)-pent-2-en-1-yl acetate C7H12O2 1.35 - 22.29 diisooctyladipate C22H42O4 0.10 0.57 others 0.78 0.41 total 4.46 0.98 Phenols 6.54 Phenol 
C6H6O 6.49 0.26 7.94 2-methoxy-phenol 
C7H8O2 1.42 2.39 7.99 phenol, 4-methyl- C7H8O 0.64 - 9.28 4-methyl-2-methoxyphenol C8H10O2 2.88 2.64 10.21 1, 2-benzenediol C6H6O2 1.86 2.34 10.54 1, 2-benzenediol, 3-methoxy- C7H8O3 1.07 0.51 10.72 phenol, 4-ethyl-2-methoxy- C9H12O2 0.63 0.46 10.97 1, 2-benzenediol, 3-methyl- C7H8O2 0.48 1.35 11.00 1, 2-benzenediol, 4-methyl- C7H8O2 1.52 - 11.21 2-methoxy-4-vinylphenol C9H10O2 2.95 4.06 11.36 2, 6-dimethoxyphenol C8H10O3 1.92 2.17 11.77 phenol, 2-methoxy-3-(2-propenyl)- C10H12O2 0.30 0.89 11.90 2-methoxy-4-(1-propenyl)phenol C10H12O2 0.19 4.18 11.91 phenol, 2-methoxy-4-propyl- C10H14O2 - 0.18 12.34 vanillin C8H8O3 0.54 1.70 12.42 2-methoxy-4-(1-propenyl)-phenol C10H12O2 0.24 - 12.58 4-ethylcatechol C8H10O2 - 0.90 12.93 3-methoxy-4-hydroxybenzoicacid C8H8O4 3.06 - 13.42 4-acetyl-2-methoxyphenol C9H10O3 0.36 0.92 13.95 homovanillic acid C9H10O4 0.65 4.53 14.35 benzoicacid, 4-hydroxy- C7H6O3 16.1 10.3 14.79 4-allyl-2, 6-dimetoxyphenol C11H14O3 5.41 4.03 15.44 siringic aldehyde C9H10O4 0.51 - 15.61 coniferylic alcohol C10H12O3 - 1.54 16.24 acetosyringone 
C10H12O4 0.57 - 16.29 p-coniferaldehyde C10H10O3 - 2.65 others 5.85 2.55 total 55.64 50.55 Others 21.89 1-phenanthrenecarboxylicac 
C21H30O2 - 0.63 24.43 (-)-pterocarpin C17H14O5 - 0.35 25.47 squalene C30H50 - 2.99 loss of column & unknowns 20.31 17.25 total 20.31 21.22 表 3 Ozawa-Flynn-Wall 计算拟合线的相关系数与活化能
Table 3 Correlation coefficient (R2) and activation energy (Ea) calculated from the Ozawa-Flynn-Wall method
Conversion x/% PKS-EMAL WS-EMAL Ea/(kJ·mol-1) R2 Ea/(kJ·mol-1) R2 20 127.92 0.980 1 244.34 0.959 9 30 135.76 0.969 3 139.75 0.993 9 40 139.71 0.949 5 134.28 0.997 9 50 140.39 0.956 7 130.98 0.998 9 60 139.88 0.961 8 128.80 0.998 9 70 153.43 0.974 9 129.68 0.998 7 80 229.16 0.985 5 224.44 0.979 1 Average 152.32 0.968 2 161.75 0.989 6 -
[1] LUPOI J S, SINGH S, PARTHASARATHI R, SIMMONS B A, HENRY R J.Recent innovations in analytical methods for the qualitative and quantitative assessment of lignin[J].Renew Sust Energy Rev, 2015, 49:871-906. doi: 10.1016/j.rser.2015.04.091 [2] 陈磊, 陈汉平, 陆强, 宋扬, 丁学杰, 王贤华, 杨海平.木质素结构及热解特性[J].化工学报, 2014, 65(9):3626-3633. http://www.cnki.com.cn/Article/CJFDTOTAL-HGSZ201409043.htmCHEN Lei, CHEN Han-ping, LU Qiang, SONG Yang, DING Xue-jie, WANG Xian-hua, YANG Hai-ping.Characterization of structure and pyrolysis behavior of lignin[J].J Chem Ind Eng, 2014, 65(9):3626-3633. http://www.cnki.com.cn/Article/CJFDTOTAL-HGSZ201409043.htm [3] CHEN L, WANG X H, YANG H P, LU Q, LI D, YANG Q, CHEN H P.Study on pyrolysis behaviors of non-woody lignins with TG-FTIR and Py-GC/MS[J].J Anal Appl Pyrolysis, 2015, 113:499-507. doi: 10.1016/j.jaap.2015.03.018 [4] SHEN D K, GU S, LUO K H, WANG S R, FANG M X.The pyrolytic degradation of wood-derived lignin from pulping process[J].Bioresour Technol, 2010, 101:6136-6146. doi: 10.1016/j.biortech.2010.02.078 [5] WANG S R, RU B, LIN H Z, SUN W X, LUO Z Y.Pyrolysis behaviors of four lignin polymers isolated from the same pine wood[J].Bioresour Technol, 2015, 182:120-127. doi: 10.1016/j.biortech.2015.01.127 [6] 武书彬, 李梦实.麦草酶解-温和酸解木质素的化学结构特性研究[J].林产化学与工业, 2006, 26(1):104-108. http://www.cnki.com.cn/Article/CJFDTOTAL-LCHX200601024.htmWU Shu-bin, LI Meng-shi.Study on chemical structure characteristics of wheat straw lignin from enzymatic hydrolysis-mild acidolysis[J].Chem Ind Forest Prod, 2006, 26(1):104-108. http://www.cnki.com.cn/Article/CJFDTOTAL-LCHX200601024.htm [7] 娄瑞.非木材纤维木素在不同热化学条件下的产物形成规律与调控途径[D].广州:华南理工大学, 2011.LOU Rui.Formation rules and pathway adjustments of pyrolysates derived from non-wood lignin under different thermochemical conditions[D].Guangzhou:South China University of Technology, 2011. [8] WEN J L, SUN S L, XUE B L, SUN R C.Structual elucidation of inhomogeneous lignins from bamboo[J].Int J Biol Macromol, 2015, 77:250-259. doi: 10.1016/j.ijbiomac.2015.03.044 [9] 娄瑞, 武书彬, 董浩亮, 吕高金.毛竹酶解/温和酸解木质素的快速热解研究[J].燃料化学学报, 2015, 43(1):42-47. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18553.shtmlLOU Rui, WU Shu-bin, DONG Hao-liang, LV Gao-jin.Fast pyrolysis of enzymatic/mild acidolysis lignin from moso bamboo[J].J Fuel Chem Technol, 2015, 43(1):42-47. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18553.shtml [10] YANG H P, YAN R, CHEN H P, LEE D H, ZHENG C G.Characteristics of hemicellulose, cellulose and lignin pyrolysis[J].Fuel, 2007, 86:1781-1788. doi: 10.1016/j.fuel.2006.12.013 [11] LOU R, WU S B.Products properties from fast pyrolysis of enzymatic/mild acidolysis lignin[J].Appl Energy, 2011, 88:316-322. doi: 10.1016/j.apenergy.2010.06.028 [12] 程辉, 余剑, 姚海琴, 许光文.木质素慢速热解机理[J].化工学报, 2013, 64(5):1757-1765.CHENG Hui, YU Jian, YAO Hai-qin, XU Guang-wen.Mechanism analysis of lignin slow pyrolysis[J].J Chem Ind Eng, 2013, 64(5):1757-1765. [13] 武宏香, 李海滨, 冯宜鹏, 王小波, 赵增立, 何方.钾元素对生物质主要组分热解特性的影响[J].燃料化学学报, 2013, 41(8):950-957. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18235.shtmlWU Hong-xiang, LI Hai-bin, FENG Yi-peng, WANG Xiao-bo, ZHAO Zeng-li, HE Fang.Effects of potassium on the pyrolysis of biomass components by TG-FTIR analysis[J].J Fuel Chem Technol, 2013, 41(8):950-957. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18235.shtml [14] MOHAMMED M A A, SALMIATON A, WAN AZLINA W A K G, AMRAN M S M, FAKHRUL-RAZI A, TAUFIQ-YAP Y H.Hydrogen rich gas from oil palm biomass as a potential source of renewable energy in Malaysia[J].Renew Sust Energ Rev, 2011, 15(2):1258-1270. doi: 10.1016/j.rser.2010.10.003 [15] 常国璋, 黄艳琴, 赖喜锐, 阴秀丽, 吴创之.棕榈壳焦CO2气化过程中反应性及结构特性研究[J].燃料化学学报, 2015, 43(8):1-8. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18675.shtmlCHANG Guo-zhang, HUANG Yan-qin, LAI Xi-rui, YIN Xiu-li, WU Chuang-zhi.Experimental study on the structure and reactivity of palm kernel shell chars during CO2 gasification[J].J Fuel Chem Technol, 2015, 43(8):1-8. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18675.shtml [16] ABNISA F, ARAMI-NIYA A, WAN DAUD W M A, SAHU J N.Characterization of bio-oil and bio-char from pyrolysis of palm oil wastes[J].Bioenergy Res, 2013, 6(2):830-840. doi: 10.1007/s12155-013-9313-8 [17] 张斌, 武书彬, 阴秀丽, 吴创之, 邱泽晶, 马隆龙.酸水解木质素的结构及热解产物分析[J].太阳能学报, 2011, 32(1):19-24. http://www.cnki.com.cn/Article/CJFDTOTAL-TYLX201101006.htmZHANG Bin, WU Shu-bin, YIN Xiu-li, WU Chuang-zhi, QIU Ze-jing, MA Long-long.Structure and pyrolysis products analysis of acid hydrolysis lignin[J].Acta Energy Sin, 2011, 32(1):19-24. http://www.cnki.com.cn/Article/CJFDTOTAL-TYLX201101006.htm [18] 何川.稀酸预处理中奇岗木质素局部化学和结构表征[D].北京:北京林业大学, 2015.HE Chuan.Topochemistry and characterization of lignin during diluted acid pretreatment of Miscanthus×giganteus[D].Beijing:Beijing Forest University, 2015. [19] SHARMA R K, WOOTEN J B, BAKIGA V L, LIN X, CHAN W G, HAJALIGOL M R.Characterization of chars from pyrolysis of lignin[J].Fuel, 2004, 83(11/12):1469-1482. [20] 路瑶, 魏贤勇, 宗志敏, 陆永超, 赵炜, 曹景沛.木质素的结构研究与应用[J].化学进展, 2013, 25(5):838-858. http://www.cnki.com.cn/Article/CJFDTOTAL-HXJZ201305021.htmLU Yao, WEI Xian-yong, ZONG Zhi-min, LU Yong-chao, ZHAO Wei, CAO Jing-pei.Structural investigationand application of lignins[J].Prog Chem, 2013, 25(5):838-858. http://www.cnki.com.cn/Article/CJFDTOTAL-HXJZ201305021.htm [21] JIANG G, NOWAKOWSKI D J, BRIDGWATER A V.Effect of the temperatureon the composition of lignin pyrolysis products[J].Energy Fuels, 2010, 24(8):4470-4475. doi: 10.1021/ef100363c [22] PENG C, ZHANG G, YUE J, XU G.Pyrolysis of lignin for phenols with alkaline additive[J].Fuel Process Technol, 2014, 124:212-221. doi: 10.1016/j.fuproc.2014.02.025 [23] 常国璋, 黄艳琴, 谢建军, 阴秀丽, 吴创之.棕榈壳热解失重特性及动力学研究[J].林产化学与工业, 2016, 36(4):31-40.CHANG Guo-zhang, HUANG Yan-qin, XIE Jian-jun, YIN Xiu-li, WU Chuang-zhi.Products characteristics and kinetic analysis of palm kernel shell pyrolysis[J].Chem Ind Forest Prod, 2016, 36(4):31-40. [24] 杨海平, 陈汉平, 晏蓉, 张世红, 郑楚光.油棕废弃物热解的TG-FTIR分析[J].燃料化学学报, 2006, 34(3):309-314. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract17001.shtmlYANG Hai-ping, CHEN Han-ping, YAN Rong, ZHANG Shi-guang, ZHENG Chu-guang.TG-FTIR analysis of palm oil wastes pyrolysis[J].J Fuel Chem Technol, 2006, 34(3):309-314. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract17001.shtml [25] DEL RIO J C, GUTIÉRREZ A, RODRÍGUEZ I M, IBARRA D, MARTINEZ A T.Composition of non-woody plant lignins and cinnamic acids by Py-GC/MS, Py/TMAH and FT-IR[J].J Anal Appl Pyrolysis, 2007, 79(1):39-46. [26] SAMMONS R J, HARPER D P, LABBE N, BOZELL J J, ELDER T, RIALS T G.Characterization of organosolv lignins using thermal and FT-IR spectroscopic analysis[J].BioResources, 2013, 8(2):2751-2767. [27] NIU S L, HAN K H, LU C M.Kinetic calculations for the thermal decomposition of calcium propionate under non-isothermal conditions[J].Chin Sci Bull, 2011, 56(12):1278-1284. doi: 10.1007/s11434-010-4065-8 -





 下载:
下载: