Preparation of β-Mo2C, Ni3Mo3N/β-Mo2C and its catalytic performance for methanation
-
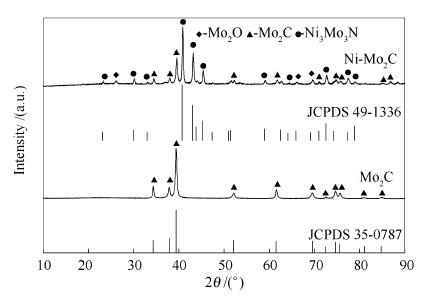
摘要: 通过焙烧钼酸铵和六次甲基四胺(HMT)生成的络合物,制备β-Mo2C。在此基础上加入Ni助剂制备了Ni3Mo3N/β-Mo2C双金属碳化物催化剂。采用XRD、SEM、HRTEM、低温氮吸附、元素分析等方法对催化剂进行了表征,考察了其合成气甲烷化反应性能。结果表明,β-Mo2C有较高的CO转化率,但CO转化率和CH4选择性分别从第10 h的75.93%和36.79%降低到了第100 h的67.41%和33.54%。因此,β-Mo2C活性不够稳定且CH4选择性较低。而Ni助剂的加入显著提高了催化剂的甲烷化活性及稳定性,使CO转化率和CH4选择性分别从第10 h的83.15%和46.64%升高到了第100 h的92.51%和57.23%。这是因为Ni助剂的加入有助于生成Ni3Mo3N,新生成的Ni3Mo3N有利于甲烷化反应。Abstract: A complexes was produced using hexamethylenetetramine(HMT) as the complexing agent of ammonium molybdate, and β-Mo2C was prepared by a simple thermal decomposition of this complexes. And then Ni was introduced and the bimetallic carbide Ni3Mo3N/β-Mo2C was prepared. The as-prepared products were characterized by XRD, low-temperature nitrogen adsorption, SEM, HRTEM, element analysis (EA), and the performances of the prepared catalysts for methanation were investigated. The results showed that the bulk molybdenum carbide exhibited high conversion of CO (xCO), but xCO and selectivity of CH4 (sCH4) on β-Mo2C decreased from 75.93% and 36.79% to 67.41% and 33.54% within 100 h. Thus the catalytic activity was not stable and sCH4 was low. The addition of Ni markedly promoted the catalyst activity and stability, xCO and sCH4 on Ni3Mo3N/β-Mo2C increased from 83.15% and 46.64% to 92.51% and 57.23% within 100h, which should be attributed to the newly produced Ni3Mo3N after Ni addition.
-
Key words:
- β-Mo2C /
- Ni3Mo3N /
- methanation
-
表 1 催化剂的孔径结构和元素分析
Table 1 Pore structure and elemental analysis of the catalysts
Sample Specific surface area A/(m2·g-1) Pore volumev/(cm3·g-1) Average pore diameter d/nm Atom content w/% Moa Nia Cb Nb Hb Oc β-Mo2C 10.26 0.0399 15.57 89.08 - 7.52 0.15 0.07 3.18 Ni-Mo2C 6.49 0.0447 27.56 69.35 20.96 2.12 1.85 0.07 5.65 a: ICP-AES; b: elemental analysis; c: calculated by the subtraction 表 2 催化剂的甲烷化性能a,b
Table 2 Performance of different catalysts
Catalyst xCO/% Rate c/(mol·hcat-1·g-1) sCO2/% Selectivity of hydrocarbon product s/% sliquid/% CH4 C2H6 C2H4 β-Mo2C 67.41 1.85 38.34 33.54 16.15 4.34 7.63 Ni/β-Mo2C 92.51 3.09 32.71 57.23 1.83 0.11 8.12 a: reaction conditions: H2/CO(mol ratio)=2.0,p=3.0MPa,T=773K,GHSV= 4100h-1
b: time on stream: 100h
c: 2mL β-Mo2C 4.64g,2mL Ni/β-Mo2C 3.81g -
[1] 付国忠, 陈超. 我国天然气供需现状及煤制天然气工艺技术和经济性分析[J]. 中外能源, 2010, 15(6): 28-34. http://www.cnki.com.cn/Article/CJFDTOTAL-SYZW201006010.htmFU Guo-zhong, CHEN Chao. NG demand and supply in China and economic and technical analysis of coal gasification technology[J]. Sino-Global Energy, 2010, 15(6): 28-34. http://www.cnki.com.cn/Article/CJFDTOTAL-SYZW201006010.htm [2] 杨春生. 煤制天然气产业发展前景分析[J]. 中外能源, 2010, 15(7): 35-40. http://www.cnki.com.cn/Article/CJFDTOTAL-SYZW201007009.htmYANG Chun-sheng. Prospects for coal gasification in China[J]. Sino-Global Energy, 2010, 15(7): 35-40. http://www.cnki.com.cn/Article/CJFDTOTAL-SYZW201007009.htm [3] 路霞, 陈世恒, 王万丽, 马紫峰. CO甲烷化Ni基催化剂的研究进展[J]. 石油化工, 2010, 39(3): 340-345. http://www.cnki.com.cn/Article/CJFDTOTAL-SYHG201003065.htmLU Xia, CHEN Shi-heng, WANG Wan-li, MA Zi-feng. Progress in Ni-based catalysts for CO methanation[J]. Petrochem Technol, 2010, 39(3): 340-345. http://www.cnki.com.cn/Article/CJFDTOTAL-SYHG201003065.htm [4] 莫欣满, 董新法, 刘其海. 纳米ZrO2负载Ni催化剂催化CO选择性甲烷化[J]. 石油化工, 2008, 37(4): 656-661.MO Xin-man, DONG Xin-fa, LIU Qi-hai. Selectivity methanation of CO over Ni-based catalysts supported on nano-Zirconia[J]. Petrochem Technol, 2008, 37(4): 656-661. [5] 罗来涛, 李松军, 邓庚凤. Sm2O3对Ni/sepiolite甲烷化催化剂的影响[J]. 燃料化学学报, 2011, 29(4): 302-304. http://www.cnki.com.cn/Article/CJFDTotal-RLHX200104002.htmLUO Lai-tao, LI Song-jun, DENG Geng-feng. Effect of samarium on Ni/sepiolite methanation catalyst[J]. J Fuel Chem Technol, 2011, 29(4): 302-304. http://www.cnki.com.cn/Article/CJFDTotal-RLHX200104002.htm [6] 田大勇, 杨霞, 秦绍东. 载体及助剂对镍基甲烷化催化剂稳定性的影响[J]. 化工进展, 2012, 31(S1): 229-231. http://www.cnki.com.cn/Article/CJFDTOTAL-HGJZ2012S1051.htmTIAN Da-yong, YANG Xia, QIN Shao-dong. Effect of supporter and promoter on stability of Ni-based methanation catalysts[J]. Chem Ind Eng Prog, 2012, 31(S1): 229-231. http://www.cnki.com.cn/Article/CJFDTOTAL-HGJZ2012S1051.htm [7] CHEN J G. Carbide and nitride over layers on early transition metal surface: Preparation, characterization and reactivities[J]. Chem Rev, 1996, 96(4): 1477-1498. doi: 10.1021/cr950232u [8] RAMANATHAN S, OYAMA S T. New catalysts for hydroprocessing: Transition metal carbides and nitrides[J]. J Phys Chem, 1995, 99(44): 16365-16372. doi: 10.1021/j100044a025 [9] CHOI J S, MAUGE F, PICHON C. Alumina-supported cobalt-molybdenum sulfide modified by tin via surface organometallic chemistry: Application to the simultaneous hydrodesulfurization of thiophenic compounds and the hydrogenation of olefins[J]. Appl Catal A: Gen, 2004, 267(2): 203-216. https://www.researchgate.net/publication/271041905_Alumina-supported_cobaltmolybdenum_sulfide_modified_by_tin_via_surface_organometallic_chemistry_application_to_the_simultaneous_hydrodesulfurization_of_thiophenic_compounds_and_the_hydrogenation_of_ol [10] MASHKINA A V. Thiophene hydrogenation to tetrahydrothiophene over tungsten sulfide catalysts[J]. Kinet Catal, 2003, 44(2): 277-282. doi: 10.1023/A:1023316831685 [11] ABE H, BELL A T. Catalytic hydrotreating of Indole, Benzothiophene and Benzofuran over molybdenum nitride[J]. Catal Lett, 1993, 18(3): 1-8. https://www.researchgate.net/publication/226691688_Catalytic_hydrotreating_of_indole_benzothiophene_and_benzofuran_over_Mo2N [12] SAJKOWSKI D J, OYAMA S T. Catalytic hydrotreating by molybdenum nitrides and molybdenum carbides[J]. Appl Catal A: Gen, 1996, 134(2): 339-349. doi: 10.1016/0926-860X(95)00202-2 [13] OSHIKAWA K, NAGAI M, OMI S. Characterization of molybdenum carbides for methane reforming by TPR, XRD, and XPS[J]. J Phys Chem B, 2001, 105(38): 9124-9131. doi: 10.1021/jp0111867 [14] WANG D, LUNSFORD J H, ROSYNEK M P. Characterization of a Mo/ZSM-5 catalyst for the conversion of methane to benzene[J]. J Catal, 1997, 169(1): 347-358. doi: 10.1006/jcat.1997.1712 [15] BLEKKAN E, GUONG P H, LEDOUX M J, GUILLE J. Isomerization of n-heptane on an oxygen-modified molybdenum carbide catalyst[J]. Ind Eng Chem Res, 1994, 33(2): 1657-1664. https://www.researchgate.net/publication/231366609_Isomerization_of_n-Heptane_on_an_Oxygen-Modified_Molybdenum_Carbide_Catalyst [16] PARK H K. A general surface propertiesand reactivity of supported and unsupported molybdenum nitride catalysts[J]. Appl Catal, 1997, 150(1): 21-35. doi: 10.1016/S0926-860X(96)00297-9 [17] KIM D. CoMo bimetallic nitrides catalysts for thiophene HDS[J]. Catal Lett, 1997, 43(1): 91-95. [18] PAUL A. Thiophene HDS over alumina-supported molybdenum nitride and carbide: Adsorption sites,catalytic activities and nature of the active surface[J]. J Catal, 1996, 164(1): 109-121. doi: 10.1006/jcat.1996.0367 [19] SCHLATTER J C, OYAMA S T. Catalytic behavior of selected transition-metal carbide, nitride and borides in the HDN of quinolin[J]. Ind Eng Chem Res, 1988, 27(9): 1648-1653. doi: 10.1021/ie00081a014 [20] LI S, LEE J S, HYEON T, SUSLICK K S. Catalytic hydrodenitrogenation of indole over molybdenum nitride and carbides with different structures[J]. Appl Catal A: Gen, 1999, 184(1): 1-9. doi: 10.1016/S0926-860X(99)00044-7 [21] SUNDARAMURTHY V, DALAI A K, ADJAYE J. Comparison of P-containing γ-Al2O3 supported Ni-Mo bimetallic carbide, nitride and sulfide catalysts for HDN and HDS of gas oils derived from Athabasca bitumen[J]. Appl Catal A: Gen, 2006, 311(1): 155-163. [22] JEONG G. HDN of pyridine over molybdenum carbide[J]. J Catal, 1995, 154(1): 33-40. doi: 10.1006/jcat.1995.1143 [23] COLLING C W, THOMPSON L T. The structure and function of supported molybdenum nitride hydrodenitrogenation catalysts[J]. J Catal, 1994, 146(1): 193-203. doi: 10.1016/0021-9517(94)90022-1 [24] MIGA K, STANCZYK K, SAYAG C, BRODZKI D, DJÉGA-MARIADASSOU G. Bifunctional behavior of bulk MoOxNy and nitrided supported NiMo catalyst in hydrodenitrogenation of indole[J]. J Catal, 1999, 183(1): 63-68. doi: 10.1006/jcat.1998.2381 [25] OZKAN U S, ZHANG L, CLARK P A. Performance and postreaction characterization of γ-Mo2N catalysts in simultaneous hydrodesulfurization and hydrodenitrogenation reactions[J]. J Catal, 1997, 172(2): 294-306. doi: 10.1006/jcat.1997.1873 [26] NAGAI M, KURAKAMI T, OMI S. Activity of carbided molybdenum-alumina for CO2 hydrogenation[J]. Catal Today, 1998, 45(1/4): 235-239. [27] NAGAI M, OSHIKAWA K, KURAKAMI T, MIYAO T, OMI S. Surface properties of carbided molybdenum-alumina and its activity for CO2 hydrogenation[J]. J Catal, 1998, 180(1): 14-23. doi: 10.1006/jcat.1998.2262 [28] LEE J S, YEOM M H, PARK K Y, NAM I S, CHUNG J S, KIM Y G, MOON S H. Preparation and benzene hydrogenation activity of supported molybdenum carbide catalysts[J]. J Catal, 1991, 128(1): 126-136. doi: 10.1016/0021-9517(91)90072-C [29] YANG S, LI C, XU J, XIN Q. In situ probing of surface sites on supported molybdenum nitride catalyst by CO adsorption[J]. Chem Commun, 1997, 127(13): 1247-1248. https://www.researchgate.net/publication/244536725_In_situ_probing_of_surface_sites_on_supported_molybdenum_nitride_catalyst_by_CO_adsorption [30] AFANASIEV P. New single source route to the molybdenum nitride Mo2N[J]. Inorg Chem, 2002, 41(21): 5317-5319. doi: 10.1021/ic025564d [31] WANG H M, LI W, ZHANG M H. New approach to the synthesis of bulk and supported bimetallic molybdenum nitrides[J]. Chem Mater, 2005, 17(12): 3262-3267. doi: 10.1021/cm047735d -





 下载:
下载: