Aqueous phase hydrogenation of levulinic acid to γ-valerolactone on supported Ru catalysts prepared by microwave-assisted thermolytic method
-
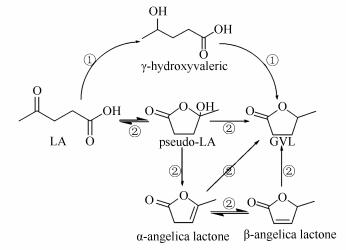
摘要: 生物质衍生物乙酰丙酸是生物质转化过程中重要的平台分子,对其进行催化加氢可以得到高附加值的产物,是连接生物质转化和石油化工的重要途径。本实验研究了无溶剂微波辅助热解法绿色制备负载型钌基催化剂,以Ru3(CO)12为金属前体,碳纳米管、椰壳活性炭和活性氧化铝为催化剂载体,该制备方法简单易操作,环保高效低能耗,不使用溶剂,避免了杂质的引入和对催化剂的污染,是一种新型负载型贵金属催化剂的制备方法。同样采取传统浸渍法制备Ru/γ-Al2O3-IM。在乙酰丙酸水相催化加氢反应中的催化活性顺序为Ru/AC > Ru/CNT ≈ Ru/FCNT > Ru/γ-Al2O3-MW ≈ Ru/γ-Al2O3-IM。比较不同反应溶液水、甲醇、乙醇、苯甲醚、环己烷和丙酮等对于乙酰丙酸催化加氢反应的影响,并通过考察反应温度、反应压力和反应物初始浓度等因素对加氢反应的影响,确定最佳实验条件为:反应温度为90℃,反应压力2.0 MPa,适宜反应物浓度为0.10 g/mL,产品GVL收率大于99%。Abstract: γ-Valerolactone (GVL), as a sustainable platform chemical, were produced through an aqueous phase hydrogenation of biomass-derived levulinic acid (LA) in the presence of supported ruthenium catalysts, in which the catalysts were prepared by solvent-free microwave-assisted thermolytic method. The effects of catalyst support, reaction media, pressure, temperature and LA initial concentration were investigated to obtain the optimum conditions for high γ-valerolactone yield. 5% Ru/AC catalyst exhibits a more superior catalytic performance compared with Ru/CNT, Ru/FCNT, Ru/γ-Al2O3-MW and Ru/γ-Al2O3-IM at 100℃ and 2.0 MPa of 0.10 g/mL LA concentration in water solution. This superior performance is attributed to the higher dispersion of metallic Ru over coconut shell activated carbon. GVL can be produced with a good yield of > 99% under optimum conditions, and has the potential to provide a green, renewable platform for biotransformation.
-
Table 1 Literature overview on LA hydrogenation in batch set-ups by using supported ruthenium catalysts
Catalyst Solvent t/℃ H2 p/MPa Time t/h LA
Con.x/%GVL
Sel.s/%Ref. Ru/C (5%) dioxane 150 5.5 2 80 92 [6] Ru/C (5%) H2O 130 1.2 2.7 99.5 86.6 [7] methanol 99 85 ethanol 76 81 1-butanol 49 82 dioxane 99 98 Ru/C (5%) H2O 180 3.0 12 100 57 [8] Ru/C (5%) methanol 130 1.2 2.7 92 99 [9] Ru/starbon(5% Ru) ethanol + H2O 100 1.0 2.2 99 5 [10] Ru/SiO2 (5%) ethanol + H2O 130 1.2 2.7 98 77 [7] Ru/Al2O3 (5%) 95 80 Ru/TiO2 (5%) 81 88 Table 2 Effect of reaction solvent upon the hydrogenation of LAa
Solvent Con.x/% Sel.s/%b By product Reaction rate/(10-3 mol·L-1·min-1)c Water 100 100 17.25 Cyclohexane 96 100 16.21 Anisole 52 41 phenol, methanol 8.82 Methanol 32 86 levulinic acid methyl ester 5.94 Ethanol 27 75 ethyl levulinate 4.29 a: reaction conditions: 0.1000 g 5% Ru/AC catalyst; cLA, 0 = 0.10 g/mL, 100 ℃, 2.0 MPa for 2 h; b : selectivity for GVL; c: the reaction rates were calculated through the slope of the profile to each reaction Table 3 Reaction rate calculated through the slope of the profile to each reaction conditiona
Entry t/℃ p/MPa cLA, 0/(g·mL-1) Reaction rate/(10-3 mol·L-1·min-1) 1 70 2.0 0.10 6.03 2 80 2.0 0.10 8.12 3 90 2.0 0.10 10.85 4 100 2.0 0.10 17.25 5 90 1.0 0.10 7.01 6 90 3.0 0.10 29.13 7 90 2.0 0.15 8.89 8 90 2.0 0.20 5.98 a: reaction conditions: 0.1000 g 5% Ru/AC catalyst, 25.00 mL aqueous solution (LA relative concentration from 1.0 to 0.6) -
[1] GIRISUTA B, JANSSEN L P B M, HEERES H J. Green chemicals:A kinetic study on the conversion of glucose to levulinic acid[J]. Chem Eng Res Des, 2006, 84(5):339-349. doi: 10.1205/cherd05038 [2] BOZELL J J. Connecting biomass and petroleum processing with a chemical bridge[J]. Science, 2010, 329(5991):522-523. doi: 10.1126/science.1191662 [3] PALKOVITS R. Pentenoic acid pathways for cellulosic biofuels[J]. Angew Chem Int Ed, 2010, 49(26):4336-4338. doi: 10.1002/anie.201002061 [4] HORVATH I T, MEHDI H, FABOS V, BODA L, MIKAIKA L T. γ-Valerolactone-a sustainable liquid for energy and carbon-based chemicals[J]. Green Chem, 2008, 10(2):238-242. doi: 10.1039/B712863K [5] LANGEANGE J P, PRICE R, AYOUB P M, LOUIS J, PETRUS L, CLARKE L, GOSSELINK H. Valeric Biofuels:A platform of cellulosic transportation fuels[J]. Angew Chem Int Ed, 2010, 49:4479-4483. doi: 10.1002/anie.201000655 [6] MANZER L E. Catalytic synthesis of α-methylene-γ-valerolactone:A biomass-derived acrylic monomer[J]. Appl Catal A:Gen, 2004, 272(1/2):249-256. doi: 10.1021/acs.chemrev.6b00314 [7] AL-SHAAL M G, WRIGHT W R H, PALKOVITS R. Exploring the ruthenium catalysed synthesis of γ-valerolactone in alcohols and utilisation of mild solvent-free reaction conditions[J]. Green Chem, 2012, 14(5):1260-1263. doi: 10.1039/c2gc16631c [8] DING D Q, WANG J J, XI J X, LIU X H, LU G Z, WANG Y Q. High-yield production of levulinic acid from cellulose and its upgrading to γ-valerolactone[J]. Green Chem, 2014, 16(8):3846-3853. doi: 10.1039/C4GC00737A [9] YAN Z P, LIN L, LIU S. Synthesis of γ-valerolactone by hydrogenation of biomass-derived levulinic acid over Ru/C catalyst[J]. Energy Fuels, 2009, 23(8):3853-3858. doi: 10.1021/ef900259h [10] LUQUE R, CLARK J H. Water-tolerant Ru-starbon® materials for the hydrogenation of organic acids in aqueous ethanol[J]. Catal Commun, 2010, 11(10):928-931. https://www.sciencedirect.com/science/article/pii/S1566736710000920 [11] ABDELRAHMAN O A, LUO H Y, HEYDEN A, ROMAN-LESHKOV Y, BOND J Q. Toward rational design of stable, supported metal catalysts for aqueous-phase processing:Insights from the hydrogenation of levulinic acid[J]. J Catal, 2015, 329:10-20. doi: 10.1016/j.jcat.2015.04.026 [12] DU X L, LIU Y M, WANG J Q, CAO Y, FAN K N. Catalytic conversion of biomass-derived levulinic acid into γ-valerolactone using iridium nanoparticles supported on carbon nanotubes[J]. Chin J Catal, 2013, 34(5):993-1001. doi: 10.1016/S1872-2067(11)60522-6 [13] XIAO C X, GOH T W, QI Z Y, GOES S, BRASHLER K, PEREZ C, HUANG W Y. Conversion of levulinic acid to γ-valerolactone over few-layer graphene-supported ruthenium catalysts[J]. ACS Catal, 2016, 6(2):593-599. doi: 10.1021/acscatal.5b02673 [14] LEONARD R H. Levulinic acid as a basic chemical raw material[J]. Ind Eng Chem, 1956, 48(8):1330-1341. doi: 10.1021/ie50560a033 [15] UPARE P P, LEE J M, HWANG D W, HALLIGUDI S B, HWANG Y K, CHANG J S J. Selective hydrogenation of levulinic acid to γ-valerolactone over carbon-supported noble metal catalysts[J]. Ind Eng Chem, 2011, 17(2):287-292. http://www.academia.edu/27144682/Selective_hydrogenation_of_levulinic_acid_to_%CE%B3-valerolactone_over_carbon-supported_noble_metal_catalysts [16] YAN Z P, LIN L, LIU S J. Synthesis of γ-valerolactone by hydrogenation of biomass-derived levulinic acid over Ru/C catalyst[J]. Energy Fuels, 2009, 23(8):3853-3858. doi: 10.1021/ef900259h [17] DENG L, LI J, LAI D M, FU Y, GUO Q X. Catalytic conversion of biomass-derived carbohydrates into γ-valerolactone without using an external H2 supply[J]. Angew Chem Int Ed, 2009, 48(35):6529-6532. doi: 10.1002/anie.v48:35 [18] DENG L, ZHAO Y, LI J, FU Y, LIAO B, GUO Q X. Conversion of levulinic acid and formic acid into γ-valerolactone over heterogeneous catalysts[J]. ChemSusChem, 2010, 3(10):1172-1175. doi: 10.1002/cssc.v3:10 [19] LIGUORI F, MORENO-MARRODAN C, BARBARO P. Environmentally friendly synthesis of γ-valerolactone by direct catalytic conversion of renewable sources[J]. ACS Catal, 2015, 5(3):1882-1894. doi: 10.1021/cs501922e [20] HEERES H, HANDRARA R, DAI C N, RASRENDRA C B, GIRISUTA B, HEERE H J. Combined dehydration/(transfer)-hydrogenation of C6-sugars (D-glucose and D-fructose) to γ-valerolactone using ruthenium catalysts[J]. Green Chem, 2009, 11(8):1247-1255. doi: 10.1039/b904693c [21] MEHDI H, FABOS V, TUBE R, BODOR A, MIKA L T, HORVATH I T. Integration of homogeneous and heterogeneous catalytic processes for a multi-step conversion of biomass:from sucrose to levulinic acid, γ-valerolactone, 1, 4-pentanediol, 2-methyl-tetrahydrofuran, and alkanes[J]. Top Catal, 2008, 48(1/4):49-54. https://es.scribd.com/.../AdvBioUsiCatRouForTheConOfBioPlaMol [22] SERRANO-RUIZ J C, BRADEN D J, WEST R M, DUMESIC J A. Conversion of cellulose to hydrocarbon fuels by progressive removal of oxygen[J]. Appl Catal B:Environ, 2010, 100(1/2):184-189. https://www.sciencedirect.com/science/article/pii/S0926337310003358 [23] BRADEN D J, HENAO C A, HELTZEL J, MARAVELIAS C C, DUMESIC J A. Production of liquid hydrocarbon fuels by catalytic conversion of biomass-derived levulinic acid[J]. Green Chem, 2011, 13(7):1755-1765. doi: 10.1039/c1gc15047b [24] NI X J, ZHANG B S, LI C, PANG M, SU D S, WILLIAMS C T, LIANG C H. Microwave-assisted green synthesis of uniform Ru nanoparticles supported on non-functional carbon nanotubes for cinnamaldehyde hydrogenation[J]. Catal Commun, 2012, 24:65-69. doi: 10.1016/j.catcom.2012.03.035 [25] DELHOMME C, WEUSTER-BOTZ D, KUHN F E. Succinic acid from renewable resources as a C4 building-block chemical-a review of the catalytic possibilities in aqueous media[J]. Green Chem, 2009, 11(1):13-26. doi: 10.1039/B810684C [26] HU X, YU J C, GONG J, LI Q, LI G. α-Fe2O3 nanorings prepared by a microwave-assisted hydrothermal process and their sensing properties[J]. Adv Mater, 2007, 19(17):2324-2329. doi: 10.1002/(ISSN)1521-4095 [27] DI X, LI C, ZHANG B S, QI J, LIANG C H. Role of Re and Ru in Re-Ru/C bimetallic catalysts for the aqueous hydrogenation of succinic acid[J]. Ind Eng Chem Res, 2017, 56(16):4672-4683. doi: 10.1021/acs.iecr.6b04875 [28] LIANG C H, DING L, LI C, PANG M, SU D S, LI W Z, WANG Y M. Nanostructured WCx/CNTs as highly efficient support of electrocatalysts with low Pt loading for oxygen reduction reaction[J]. Energy Environ Sci, 2010, 3(8):1121-1127. doi: 10.1039/c001423k [29] LANDRY C C, BARRON A R. The synthesis of polycrystalline chalcopyrite semiconductors by microwave irradiation[J]. Science, 1993, 260(5114):1653-1655. doi: 10.1126/science.260.5114.1653 [30] SATISHKUMAR B C, GOVINDARAJ A, MOFOKENG J, SUBBANNA G N, RAO C N R J. Novel experiments with carbon nanotubes:Opening, filling, closing and functionalizing nanotubes[J]. J Phys B:At Mol Opt Phys, 1996, 29(21):4925-4934. doi: 10.1088/0953-4075/29/21/006 [31] LU J S, Effect of surface modifications on the decoration of multi-walled carbon nanotubes with ruthenium nanoparticles[J]. Carbon, 2007, 45(8):1599-1605. doi: 10.1016/j.carbon.2007.04.013 [32] HENGNE A M, RODE C V. Cu-ZrO2 nanocomposite catalyst for selective hydrogenation of levulinic acid and its ester to γ-valerolactone[J]. Green Chem, 2012, 14(4):1064-1072. doi: 10.1039/c2gc16558a -





 下载:
下载: