Preparation, characterization and CO2 adsorption of ion exchange resin supported solid amine adsorbents
-
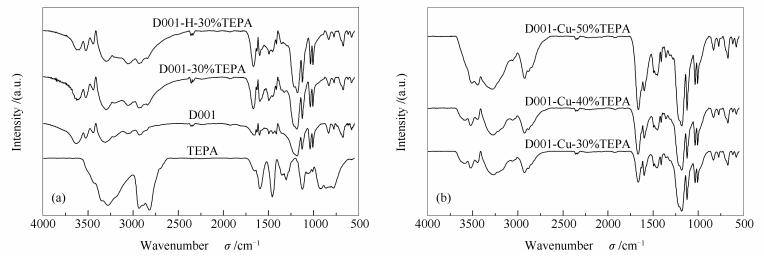
摘要: 以离子交换树脂(D001)为载体,四乙烯五胺(TEPA)为改性剂,采用三种不同的方法制备了一系列固态胺吸附剂。采用N2吸附-脱附、傅里叶变换红外光谱(FT-IR)、热重分析(TGA)等手段对吸附剂进行表征。在固定床反应器中考察了TEPA负载量、吸附温度、进气流量和CO2分压等因素对CO2吸附性能的影响。结果表明,配位法制得的固态胺吸附剂分散性和稳定性较好,且在TEPA负载量为40%,吸附温度为65℃,进气流量为40 mL/min时有最大CO2吸附量达4 mmol/g。经过10次吸附-脱附循环实验后,CO2吸附量下降3.98%。热力学、动力学研究结果表明,CO2吸附是物理吸附和化学吸附的结果。Abstract: A series of solid amine adsorbents were prepared by using three different preparation methods with ion exchange resin (D001) as carrier and with tetraethylenepentamine (TEPA) as modifier. The sorbents were characterized by nitrogen adsorption/desorption, Fourier transform infrared spectroscopy (FT-IR) and thermogravimetric analysis (TGA) techniques. The effects of TEPA loadings, adsorption temperatures, influent gas flow rates and CO2 partial pressure on the CO2 adsorption capacity in a fixed bed reactor were investigated. The results show that the solid amine adsorbent prepared by the coordination method has a better dispersibility and stability, and the maximum CO2 adsorption capacity is 4 mmol/g when the TEPA loading is 40%, the adsorption temperature is 65 ℃ and the influent gas flow rate is 40 mL/min. The amount of CO2 adsorption only decreases by 3.98% and remains almost unchanged after 10 cycles of desorption and desorption. The study of thermodynamics and kinetics indicates that the adsorption mechanism is dominated by both chemical and physical adsorption.
-
Key words:
- ion exchange resin /
- tetraethylenepentamine /
- CO2 adsorption
-
表 1 不同制备方法制得的吸附剂的孔结构参数
Table 1 Pore structural parameters of adsorbent with different preparation methods

表 2 在不同温度下CO2分压对D001-Cu-40%TEPA吸附性能的影响
Table 2 Effect of CO2 partial pressure on the adsorption performance of D001-Cu-40%TEPA

表 3 D001-Cu-40%TEPA对CO2的等量吸附热
Table 3 Isosteric heat of CO2 adsorption on D001-Cu-40%TEPA

表 4 D001-Cu-40%TEPA的CO2吸附动力学模型拟合参数
Table 4 Parameters of kinetic models for CO2 adsorption of D001-Cu-40%TEPA

-
[1] TSENG R L, WU F C, JUANG R S. Adsorption of CO2 at atmospheric pressure on activated carbons prepared from melamine-modified phenol-formaldehyde resins[J]. Sep Purif Technol, 2015, 140(1):53-60. http://www.sciencedirect.com/science/article/pii/S1383586614006972 [2] NILANTHA P W, MIETEK J. Activated carbon spheres for CO2 adsorption[J]. ACS Appl Mater Interfaces, 2013, 5(2):1849-1855. doi: 10.1021/am400112m [3] ARUNKUMAR S, ZHAO A, GEORGE K H, PARTHA S, RAJENDER G. Post-combustion CO2 capture using solid Sorbents:A review[J]. Ind Eng Chem Res, 2012, 51(10):1438-1463. doi: 10.1021/ie200686q [4] FIGUEROA J D, FOUT T, PLASYNSKI S, MCILVERIED H, SRIVASTAVA R D. Advances in CO2capture technology-The U.S. department of energy's carbon sequestration program:A review[J]. Int J Green Gas Con, 2008, 2(7):9-20. http://www.sciencedirect.com/science/article/pii/S1750583607000941 [5] WANG X, GUO Q J, KONG T T. Tetraethylenepentamine-modified MCM-41/silica gel with hierarchical mesoporous structure for CO2capture[J]. Chem Eng J, 2015, 273(3):472-480. http://www.sciencedirect.com/science/article/pii/s1385894715004258 [6] KHATRI R A, CHUANG S C, SONG Y. Thermal and chemical stability of regenerable solid amine sorbent for CO2 capure[J]. Energy Fuels, 2006, 20(4):1514-1520. doi: 10.1021/ef050402y [7] 刘之琳, 腾阳, 张锴, 曹晏, 潘伟平.不同有机胺修饰MCM-41的CO2吸附性能和热稳定性[J].燃料化学学报, 2013, 41(4):469-476. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18168.shtmlLIU Zhi-lin, TENG Yang, ZHANG Kai, CAO Yan, PAN Wei-ping. The CO2adsorption and thermal stability of MCM-41 modified by different organic amines[J]. J Fuel Chem Technol, 2013, 41(4):469-476. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18168.shtml [8] LEE S, FILBURN T P, GRAY M, PARK J W, SONG H J. Screening test of solid amine sorbents for CO2 capture[J]. Ind Eng Chem Res. 2008, 47(8):7419-7423. doi: 10.1021/ie8006984 [9] GRAY M L, HOFFMAN J S, HREHA D C, FAUTH D J, HEDGES S W, CHAMPAGNE K J, PENNLINE H W. Parametric study of solid amine sorbents for the capture of carbon dioxide[J]. Energy Fuels, 2009, 23(9):4840-4844. doi: 10.1021/ef9001204 [10] ALESI W R, KITCHIN J R. Evaluation of a primary amine-functionalized ion-exchange resin for CO2 capture[J]. Ind Eng Chem Res, 2012, 51(5):6907-6915. doi: 10.1021/ie300452c [11] 张学诗, 刘新民.改性分子筛二氧化碳捕集材料的制备及其性能[J].环境工程学报, 2015, 9(10):4995-4999. doi: 10.12030/j.cjee.20151060ZHANG Xue-shi, LIU Xin-min. Preparation and performance of modified molecular sieve for carbon dioxide capture[J]. J Environ Eng, 2015, 9(10):4995-4999. doi: 10.12030/j.cjee.20151060 [12] HU J X, WANG G C, JIANG T. Preparation and performance of sulphonated polystyrene[J]. Colloid Polym Sci, 2012, 30(1):36-38. http://www.whxb.pku.edu.cn/EN/abstract/abstract26737.shtml [13] WANG X, CHEN L L, GUO Q J. Development of hybrid amine-functionalized MCM-41 sorbents for CO2 capture[J]. Chem Eng J, 2015, 260(9):573-581. http://www.sciencedirect.com/science/article/pii/S1385894714011723 [14] CHEN Z H, DENG S B, WEI H R, WANG B, HUANG J, YU G. Polyethylenimine-impregnated resin for high CO2 adsorption:An efficient adsorbent for capture from simulated flue gas and ambient air[J]. ACS Appl Mater Interfaces, 2013, 5(6):6937-6945. http://www.ncbi.nlm.nih.gov/pubmed/23808655 [15] 陈琳琳, 王霞, 郭庆杰.四乙烯五胺修饰介孔硅胶吸附CO2性能的研究[J].燃料化学学报, 2015, 43(1):108-115. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18563.shtmlCHEN Lin-lin, WANG Xia, GUO Qing-jie. Study on CO2 adsorption properties of tetraethylenepentamine modified mesoporous silica gel[J]. J Fuel Chem Technol, 2015, 43(1):108-115. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18563.shtml [16] MEILS S D, WANG S X, MARTHA A, ARLLANO T, ROBERT J F. CO2 utilization with a novel dual function material (DFM) for capture and catalytic conversion to synthetic natural gas:An update[J]. J CO2 Util, 2016, 15(9):65-71. http://www.sciencedirect.com/science/article/pii/S2212982016300695 [17] HUANG H Y, YANG R T, CHINN D, MUNSON C L. Amine-grafted MCM-48 and silica xerogel as superior sorbents for acidic gas removal from natural gas[J]. Ind Eng Chem Res, 2003, 42(12):2427-2433. doi: 10.1021/ie020440u [18] RODRIGO S G, ABDELHAMID S. Modeling adsorption of CO2 on amine-functionalized mesoporous silica. 2:Kinetics and breakthrough curves[J]. Chem Eng J, 2010, 161(7):182-190. [19] LOGANATHAN S, TIKMANI M, EDUBILLI S. CO2 adsorption kinetics on mesoporous silica under wide range of pressure and temperature[J]. Chem Eng J, 2014, 256(11):1-8. http://www.sciencedirect.com/science/article/pii/S1385894714008407 [20] ALIAKBAR H G, ABDELHAMID S. CO2 capture on polyethylenimine-impregnated hydrophobic mesoporous silica:Experimental and kinetic modeling[J]. Chem Eng J, 2011, 173(1):72-79. doi: 10.1016/j.cej.2011.07.038 -





 下载:
下载: