Effect of alkali modification to ZSM-5 zeolite on the aromatization of methanol
-
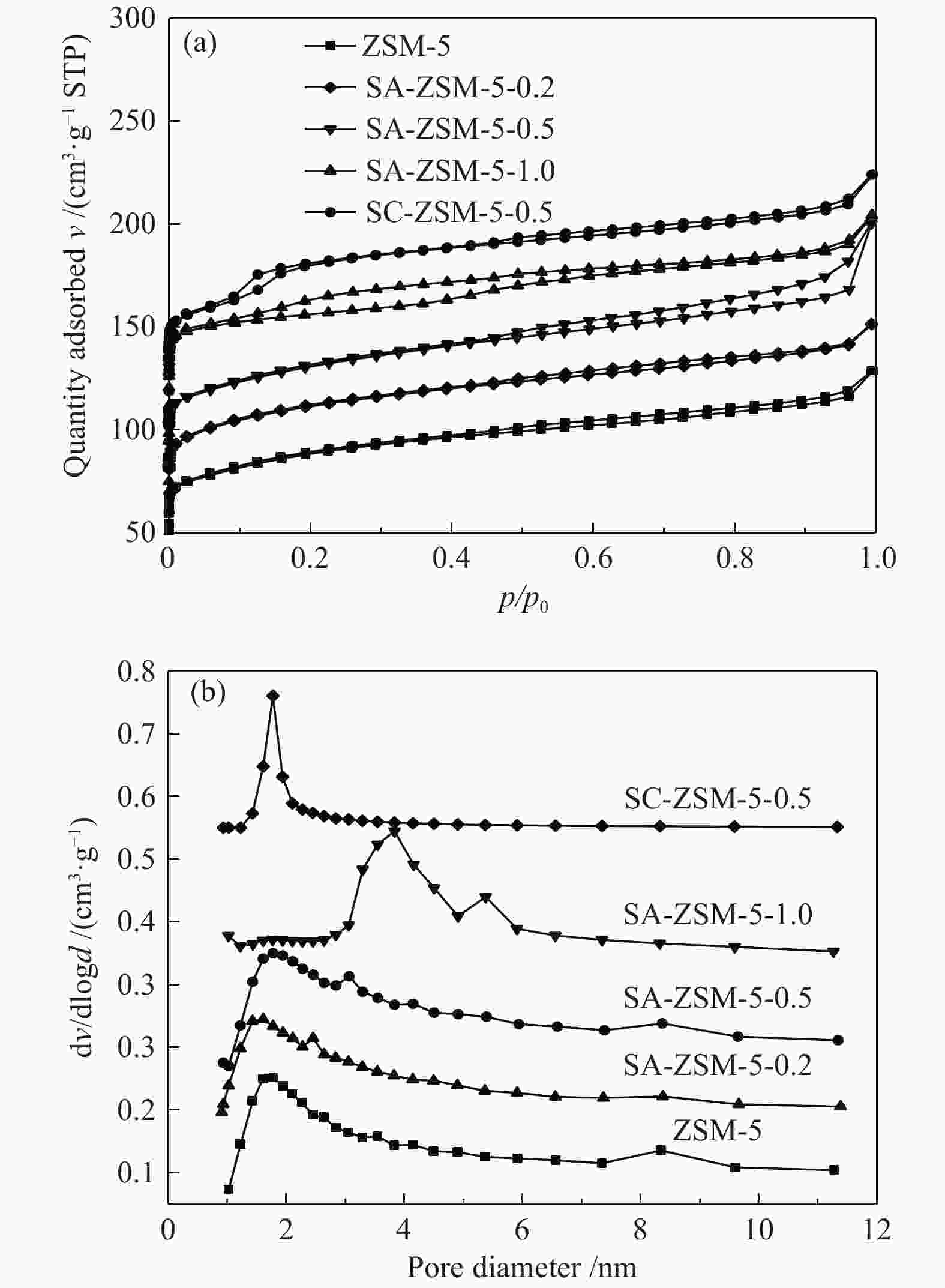
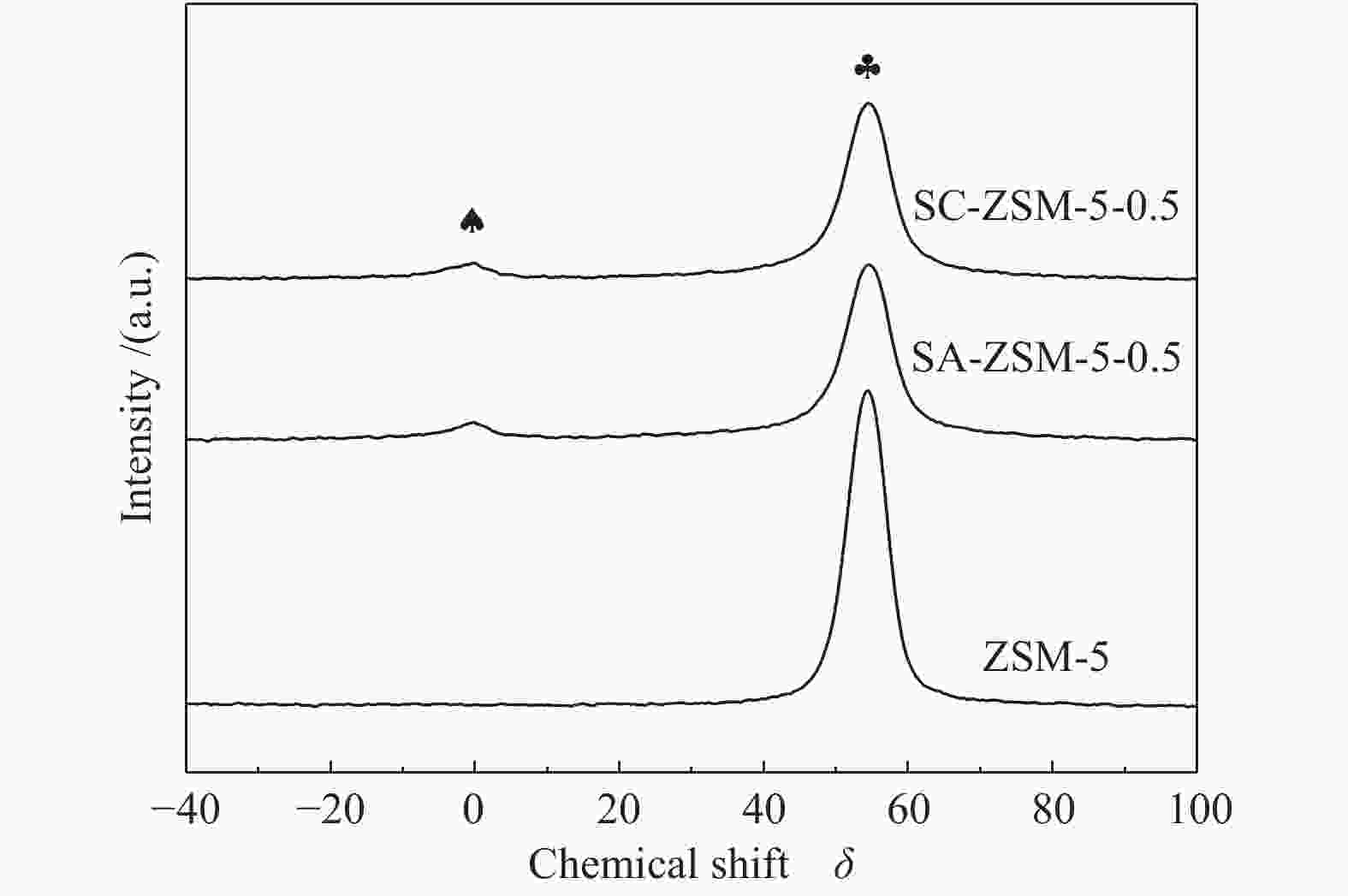
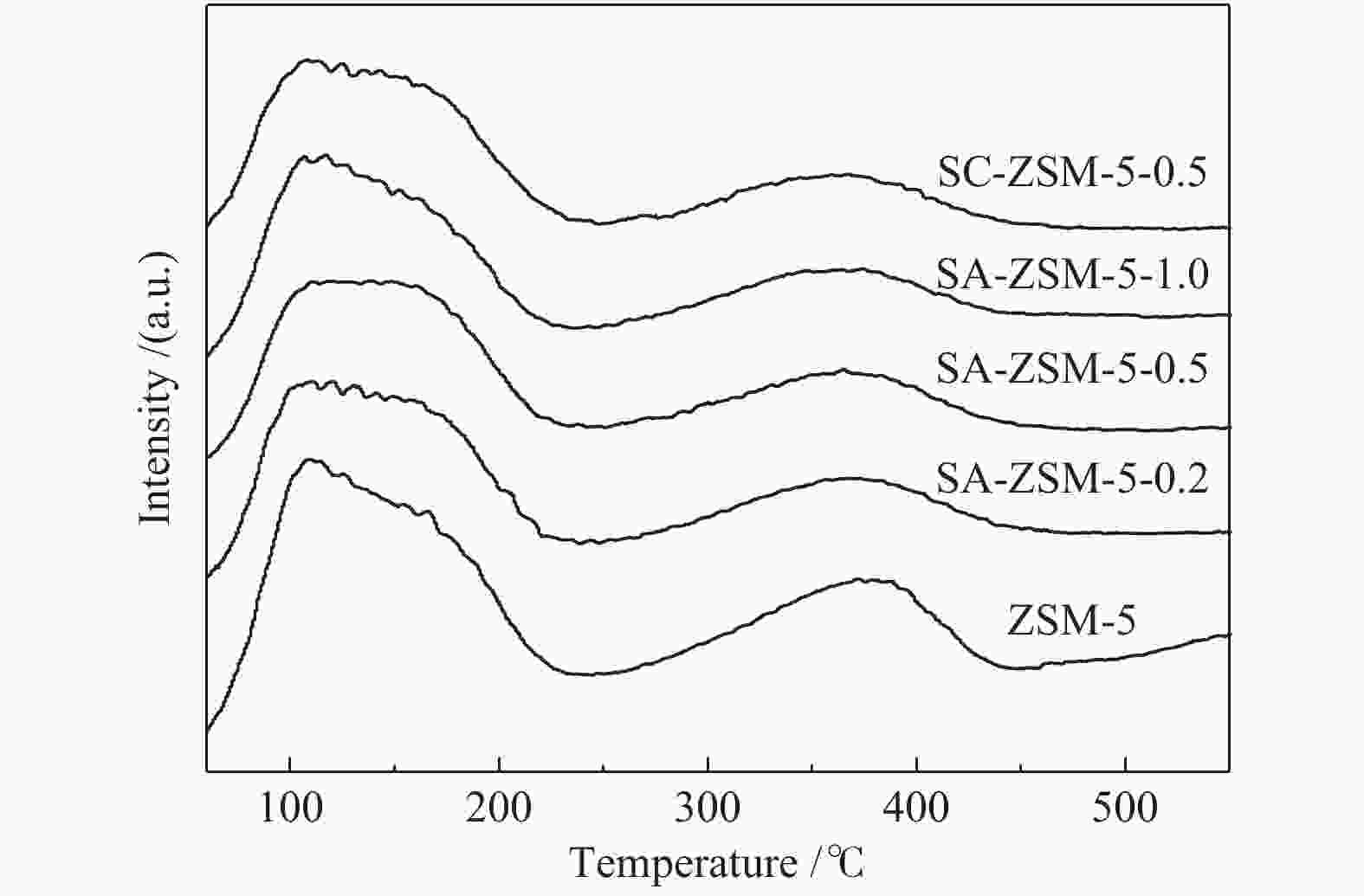
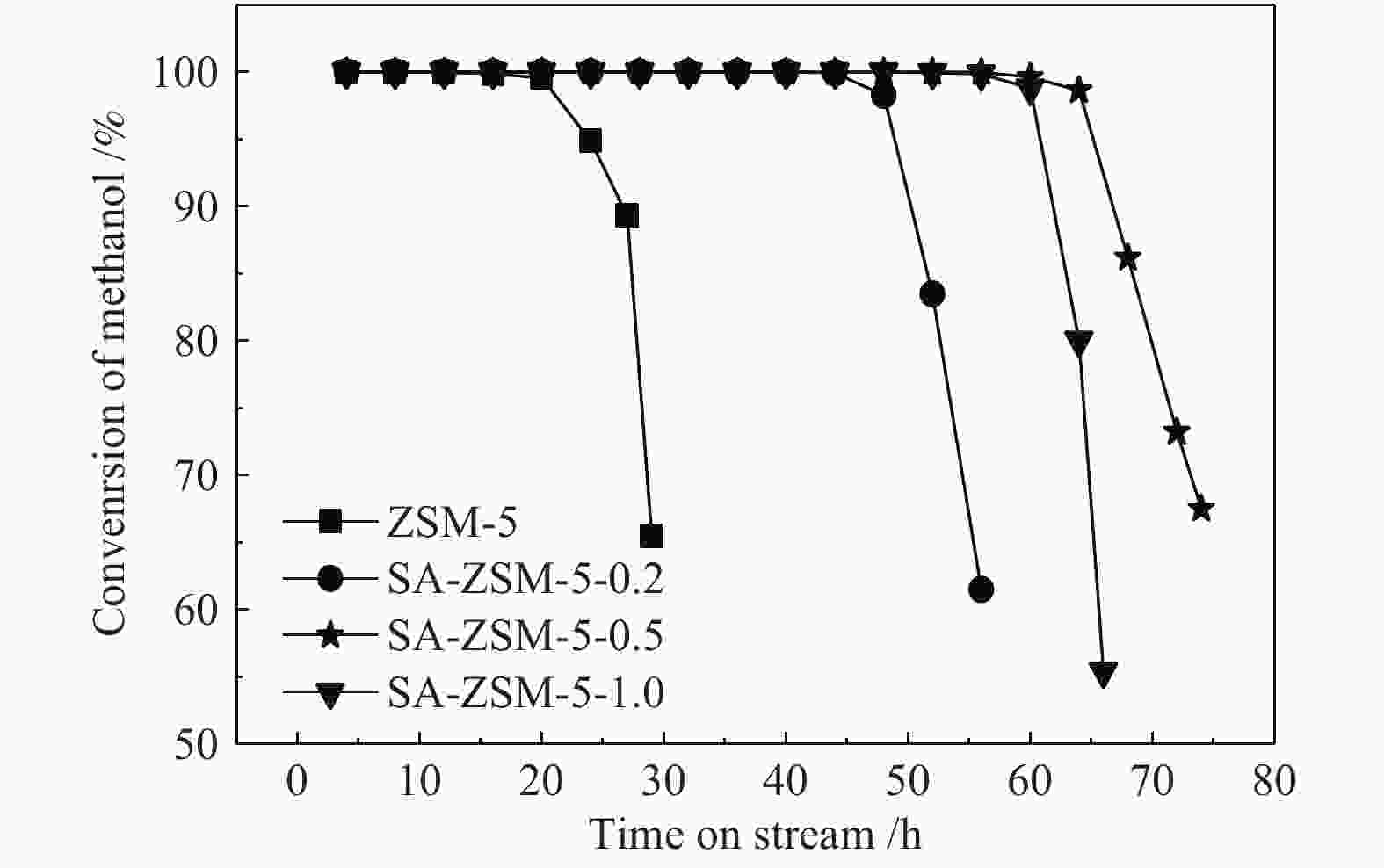
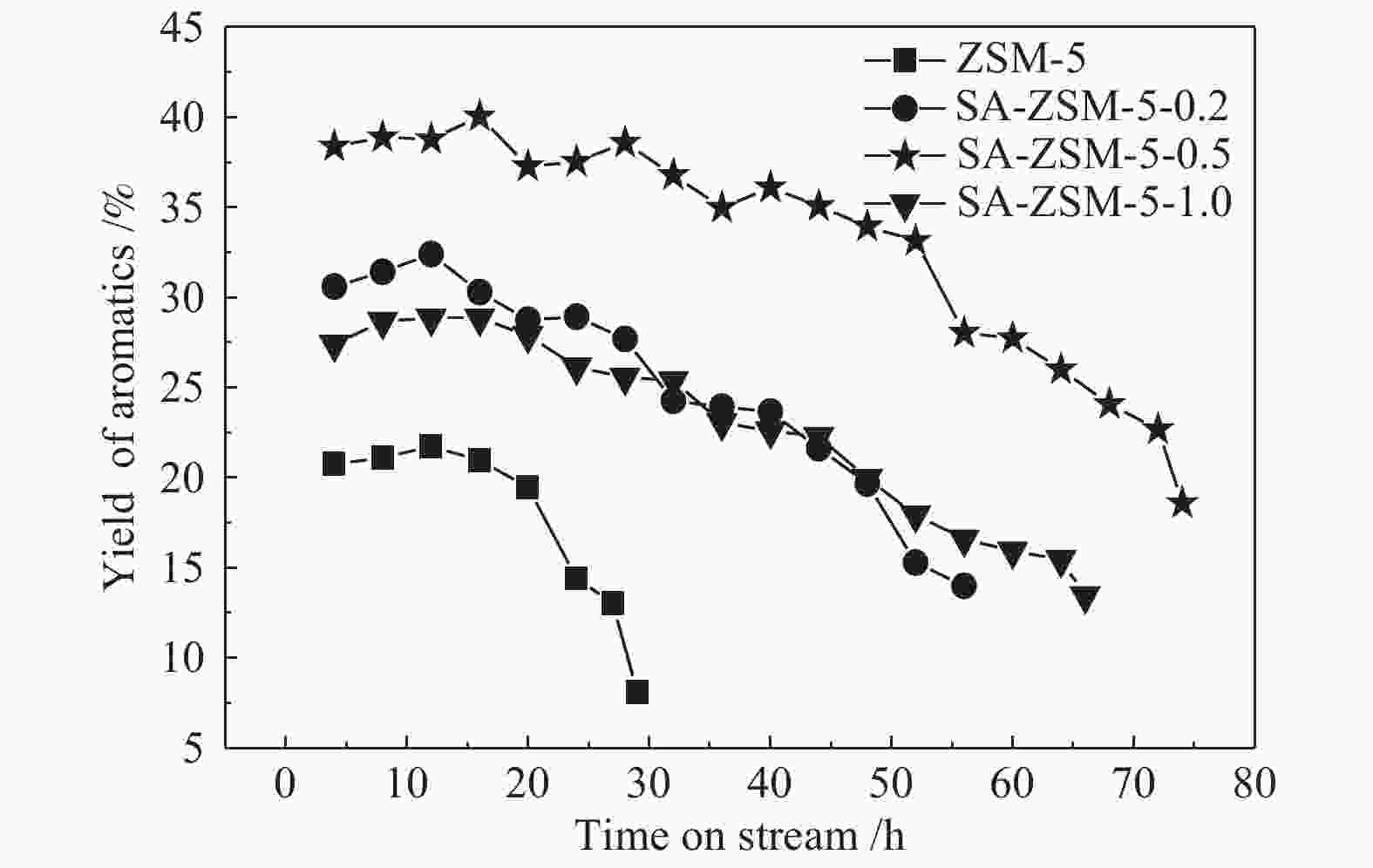
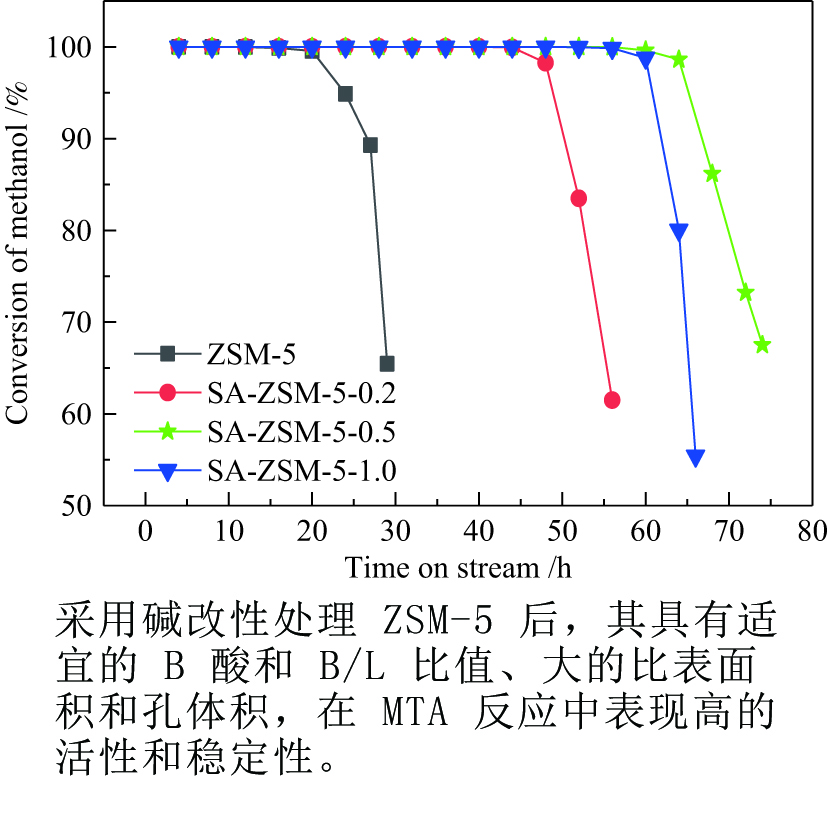
摘要: 利用乙酸钠和柠檬酸钠对ZSM-5分子筛进行脱硅改性处理,并通过XRD、SEM、NH3-TPD、27Al MAS NMR、吡啶吸附红外光谱和N2吸附-脱附等表征手段对ZSM-5分子筛结构、酸量、比表面积及孔体积等物化性质进行表征分析。结果表明,碱改性使分子筛的孔径增加且形成合适的微-介孔结构,同时,使L酸量和B酸量明显降低。当乙酸钠溶液浓度为0.5 mol/L时,改性的分子筛在形成大量介孔结构的同时具有合适的B/L值,使催化剂的寿命延长并展现出最优的催化性能。与微孔ZSM-5相比,使用寿命由20 h延长到74 h,芳烃最高产率从20.97%提高到40.05%。Abstract: ZSM-5 zeolites were modified by desilication with sodium acetate and sodium citrate. The physicochemical properties of ZSM-5 zeolites, such as crystal structure, acid content, surface area and pore volume, were characterized by XRD, SEM, NH3-TPD, 27Al MAS NMR, pyridine adsorption infrared spectroscopy and N2 adsorption-desorption isotherms. The results indicate that pore size of the zeolite increases and mesoporous structures are formed by alkali modification, and the amount of Lewis and Brönsted acid contents decreases obviously. When the concentration of sodium acetate solution is 0.5 mol/L, the modified zeolite has a suitable B/L value for the aromatization of methanol while forming a large number of mesoporous structures. Compared with microporous ZSM-5, the catalyst life is increased from 20 h to 74 h, and the highest yield of aromatics is increased from 20.97% to 40.05%.
-
Key words:
- ZSM-5 zeolite /
- alkali modified /
- mesoporous /
- aromatization of methanol
-
表 1 ZSM-5分子筛样品的相对结晶度和硅铝比
Table 1 Relative crystallinity and n(SiO2)/n(Al2O3) of ZSM-5 zeolite samples
Sample Relative crystallinity /% n(SiO2)/n(Al2O3) SC-ZSM-5-0.5 99.61 70 SA-ZSM-5-1.0 99.66 65 SA-ZSM-5-0.5 99.58 73 SA-ZSM-5-0.2 99.99 78 ZSM-5 100 81 表 2 ZSM-5分子筛样品的结构性质
Table 2 Structural properties of ZSM-5 zeolite samples
Catalyst SBET/
(m2·g−1)Sexter/
(m2·g−1)vtotal/
(cm3·g−1)vmicro/
(cm3·g−1)SC-ZSM-5-0.5 348 89 0.25 0.11 SA-ZSM-5-1.0 351 86 0.28 0.08 SA-ZSM-5-0.5 359 94 0.26 0.09 SA-ZSM-5-0.2 341 82 0.22 0.11 ZSM-5 321 54 0.20 0.12 表 3 ZSM-5分子筛样品的酸性和酸量分布
Table 3 Acidity and acidity distribution of ZSM-5 zeolite samples
Catalyst Relative
acid amountsAcid amounts/(mmol·g−1) B/L strong acidity weak acidity L B SC-ZSM-5-0.5 0.628 0.712 0.24 1.19 4.96 SA-ZSM-5-1.0 0.666 0.733 0.30 1.14 3.80 SA-ZSM-5-0.5 0.684 0.718 0.27 1.31 4.85 SA-ZSM-5-0.2 0.745 0.773 0.41 1.97 4.81 ZSM-5 1 1 3.35 5.37 1.60 note:the relative acid amount was obtained from the NH3-TPD curve; the acid amount data were obtained from a Py-FTIR spectrum at 200 ℃ -
[1] CONTE M, LOPEZ-SANCHEZ J A, QIAN H, MORGAN D J, RYABENKOVA Y, BARTLEY J K, CARLEY A F, TAYLOR S H, KIELY C J, KHALID K. Modified zeolite ZSM-5 for the methanol to aromatics reaction[J]. Catal Sci Technol,2011,2(1):105−112. [2] WANG F, XIAO W, GAO L, XIAO G. The growth mode of ZnO on HZSM-5 substrates by atomic layer deposition and its catalytic property in the synthesis of aromatics from methanol[J]. Catal Sci Technol,2016,6(9):3074−3086. [3] KUI S, WEIZHONG Q, NING W, CHANG S, FEI W. Fabrication of c-axis oriented ZSM-5 hollow fibers based on an in situ solid-solid transformation mechanism[J]. Jam Chem Soc,2013,135(41):15322−15325. [4] 梁晓彤, 范晶晶. ZSM-5分子筛改性方法概述[J]. 科技风,2019,(34):162.LIANG, Xiao-tong, FAN Jing-jing. Overview of modification methods of ZSM-5 molecular sieve[J]. Technol Wind,2019,(34):162. [5] LI J, CHAO H, KAI T, HAO X, ZHU Z, HU Z H. CO2 atmosphere-enhanced methanol aromatization over the NiO-HZSM-5 catalyst[J]. RSC Adv,2014,4(84):44377−44385. [6] BAKARE I A, MURAZA O, YAMANI Z H, YOSHIOKA M, YOKOI T. Conversion of methanol to olefins over Al-rich ZSM-5 modified with alkaline earth metal oxides[J]. Catal Sci Technol,2016,6(21):7852−7859. [7] LI G, VASSILEV P, SANCHEZ-SANCHEZ M, LERCHER J A, HENSEN E J M, PIDKO E A. Stability and reactivity of copper oxo-clusters in ZSM-5 zeolite for selective methane oxidation to methanol[J]. J Catal,2016,338:305−312. [8] YOUSHENG T, HIROFUMI K, KATSUMI K. ZSM-5 monolith of uniform mesoporous channels[J]. J Am Chem Soc,2003,125(20):6044−6045. [9] KAUR B, TUMMA M, SRIVASTAVA R. Transition-metal-exchanged nanocrystalline ZSM-5 and metal-oxide-incorporated SBA-15 catalyzed reduction of nitroaromatics[J]. Ind Eng Chem Res,2013,52(33):11479−11487. [10] AVELINO C, DÍAZ-CABANAS M J, JOAQUíN M T, FERNANDO R, JORDI R. A large-cavity zeolite with wide pore windows and potential as an oil refining catalyst[J]. Nature,2002,418(6897):514−517. [11] 杨福文, 张波. 一种ZSM-5分子筛的环保合成方法[J]. 化工管理,2020,(16):83−84. doi: 10.3969/j.issn.1008-4800.2020.16.049Yang Fu-wen, ZHANG Bo. An environmentally friendly synthesis method of ZSM-5 molecular sieve[J]. Chem Ind Manage,2020,(16):83−84. doi: 10.3969/j.issn.1008-4800.2020.16.049 [12] PAILLAUD J L, HARBUZARU B, PATARIN J, BATS N. Extra-large-pore zeolites with two-dimensional channels formed by 14 and 12 rings[J]. Science,2004,304(5673):990−992. [13] BJØRGEN, MORTEN, AKYALCIN, OLSBYE, UN NI, BENARD, SANDRINE, KOLBOE, STEIN. Methanol to hydrocarbons over large cavity zeolites: Toward a unified description of catalyst deactivation and the reaction mechanism[J]. J Catal,2010,275(1):170−180. [14] 黄世英, 熊晓云. 多级孔分子筛的合成研究进展[J]. 工业催化,2019,27(5):16−21. doi: 10.3969/j.issn.1008-1143.2019.05.004HUANG Shi-ying, XIONG Xiao-yun. Advances in synthesis of multistage pore molecular sieves[J]. Ind Catal,2019,27(5):16−21. doi: 10.3969/j.issn.1008-1143.2019.05.004 [15] 李晶, 王迪. 介孔MCM-41的合成及改性研究进展[J]. 四川化工,2019,22(2):12−15. doi: 10.3969/j.issn.1672-4887.2019.02.005LI Jing, WANG Di. Progress in synthesis and modification of mesoporous MCM-41[J]. Sichuan Chem Ind,2019,22(2):12−15. doi: 10.3969/j.issn.1672-4887.2019.02.005 [16] LIU B, CHAO L, REN Y, TAN Y, XI H, YU Q. Direct synthesis of mesoporous ZSM-5 zeolite by a dual-functional surfactant approach[J]. Chem Eng J,2012,210(6):96−102. [17] LI J, MIAO P, LI Z, HE T, HAN D, WU J, WANG Z, WU J, LI J, MIAO P. Hydrothermal synthesis of nanocrystalline H[Fe, Al]ZSM-5 zeolites for conversion of methanol to gasoline[J]. Energ Convers Manage,2015,93:259−266. [18] EGEBLAD K, CHRISTENSEN C H, KUSTOVA M, CHRISTENSEN C H. Templating mesoporous zeolites[J]. Chem Mater,2008,20(3):946−960. [19] JAVIER P R, CHRISTENSEN C H, KRESTEN E, CHRISTENSEN C H, GROEN J C. Hierarchical zeolites: enhanced utilisation of microporous crystals in catalysis by advances in materials design[J]. Chem Soc Rev,2008,37(11):2530−2542. [20] BOISEN A, SCHMIDT I, CARLSSON A, DAHL S, BRORSON M, JACOBSEN C J. TEM stereo-imaging of mesoporous zeolite single crystals[J]. Chem Commun,2003,9(8):958−959. [21] WEI F, SNYDER M A, SANDEEP K, PYUNG-SOO L, WON CHEOL Y, MCCORMICK A V, PENN R, L EE, ANDREAS S, MICHAEL T. Hierarchical nanofabrication of microporous crystals with ordered mesoporosity[J]. Nat Mater,2008,7(12):984−991. [22] ZHU H, LIU Z, WANG Y, KONG D, XIE Z. Nanosized CaCO3 as hard template for creation of intracrystal pores within silicalite1 crystal[J]. Chem Mater,2008,20(3):1134−1139. [23] YAO J, YI H, WANG H. Controlling zeolite structures and morphologies using polymer networks[J]. J Mater Chem,2010,20(44):9827−9831. [24] SERRANO D P, ESCOLA J M, PIZARRO P. Synthesis strategies in the search for hierarchical zeolites[J]. Chem Soc Rev,2013,42(9):4004−4035. [25] LYNCH J, RAATZ F, DUFRESNE P. Characterization of the textural properties of dealuminated HY forms[J]. Zeolites,1987,7(4):333−340. [26] GROEN J C and MOULIJN J A. Desilication: On the controlled generation of mesoporosity in MFI zeolites[J]. J Mater Chem,2006,16(22):2121−2131. [27] VERBOEKEND D, MITCHELL S, MILINA M, GROEN J C, PEREZ-RAMIREZ J. Full compositional flexibility in the preparation of mesoporous MFI zeolites by desilication[J]. J Phys Chem C,2011,115(29):14193−14203. [28] 赵玉琦, 张弦, 刘晓飞, 郭雅新, 董若楠和常四良. 碱改性法制备微孔-介孔ZSM-5分子筛的研究进展[J]. 中国石油和化工标准与质量,2017,37(15):104−106. doi: 10.3969/j.issn.1673-4076.2017.15.053ZHAO Yu-qi, ZHANG Xuan, LIU Xiao-fei, GUO Ya-xin, DONG Ruo-nan, CHANG Si-liang. Preparation of microporous mesoporous ZSM-5 zeolite by alkali modification[J]. China Pet Chem Ind Standard Qual,2017,37(15):104−106. doi: 10.3969/j.issn.1673-4076.2017.15.053 [29] SU L, LIN L, ZHUANG J, WANG H, LI Y, SHEN W, XU Y, BAO X. Creating mesopores in ZSM-5 zeolite by alkali treatment: A new way to enhance the catalytic performance of methane dehydroaromatization on Mo/HZSM-5 catalysts[J]. Catal Lett,2003,91(3/4):155−167. [30] 魏民, 孔飞飞, 丁越野和刘冬梅. 碱处理法制备微介孔ZSM-5及其加氢脱硫性能的研究[J]. 石油炼制与化工,2015,46(10):61−66. doi: 10.3969/j.issn.1005-2399.2015.10.012Wei Min, KONG Fei-fei, DING Yue-ye, LIU Dong-mei. Preparation of micromesoporous ZSM-5 by alkali treatment and its hydrodesulfurization performance[J]. Pet Ref Chem Ind,2015,46(10):61−66. doi: 10.3969/j.issn.1005-2399.2015.10.012 [31] 马健, 刘冬梅, 魏民, 王海彦, 王坤, 张晶卫. Na2CO3溶液处理对Ni-Mo/HZSM-5分子筛硫醚化催化性能的影响[J]. 燃料化学学报,2014,42(9):1128−1134. doi: 10.3969/j.issn.0253-2409.2014.09.014MA Jian, LIU Dong-mei, WEI Min, WANG Hai-yan, WANG Kun, ZHANG Jing-wei. Effect of Na2CO3 solution treatment on the catalytic performance of thioetherification of Ni-MO /HZSM-5 zeolite[J]. J Fuel Chem Technol,2014,42(9):1128−1134. doi: 10.3969/j.issn.0253-2409.2014.09.014 [32] 赵岑, 刘冬梅, 魏民, 孙志岩, 王海彦. 多级孔ZSM-5分子筛的制备及催化噻吩烷基化性能研究[J]. 燃料化学学报,2013,41(10):1256−1261.ZHAO Cen, LIU Dong-mei, WEI Min, SUN Zhi-yan, WANG Hai-yan. Preparation and catalytic alkylation of thiophene using multistage pore ZSM-5 zeolite[J]. J Fuel Chem Tehnol,2013,41(10):1256−1261. [33] GROEN J C, MOULIJN J A, PÉREZ-RAMíREZ J. Decoupling mesoporosity formation and acidity modification in ZSM-5 zeolites by sequential desilication-dealumination[J]. Microporous Mesoporous Mater,2005,87(2):153−161. [34] GROEN J C, PEFFER L A A, MOULIJN J A, PÉREZ-RAMíREZ J. Mesoporosity development in ZSM-5 zeolite upon optimized desilication conditions in alkaline medium[J]. Colloids Surfaces A,2004,241(1):53−58. [35] FREUDE D, HAASE J (1993). Quadrupole effects in solid-state nuclear magnetic resonance[J]. Springer Berlin,1993,:74−79. [36] BRUNNER E, ERNST H, FREUDE D, FRöHLICH T, HUNGER M, PFEIFER H. Magic-angle-spinning NMR studies of acid sites in zeolite H-ZSM-5[J]. J Catal,1991,22(1):34−41. [37] SONG Y Q, FENG Y L, LIU F, KANG C L, ZHOU X L, XU L Y, YU G X. Effect of variations in pore structure and acidity of alkali treated ZSM-5 on the isomerization performance[J]. J Mol Catal A: Chem,2009,310(1):130−137. [38] LIU B, CHAI Y, LI Y, WANG A, LIU Y, LIU C. Effect of sulfidation atmosphere on the performance of the CoMo/γ-Al2O3 catalysts in hydrodesulfurization of FCC gasoline[J]. App Catal A: Gen,2014,471(10):70−79. [39] 孙巾茹, 王颖, 马强, 李滨, 赵玉, 田雨, 王虹, 迟姚玲, 李翠清, 宋永吉. Ag(x)/ZSM-5催化剂的CH4-SCR脱硝性能[J]. 燃料化学学报,2020,48(2):197−204. doi: 10.3969/j.issn.0253-2409.2020.02.009SUN Jin-ru, WANG Ying, MA Qiang, LI Bin, ZHAO Yu, TIAN Yu, WANG Hong, CHI Yao-ling, LI Cui-qing, SONG Yong-ji. Denitrification performance of Ag(X)/ZSM-5 catalyst with CH4-SCR[J]. J Fuel Chem Technol,2020,48(2):197−204. doi: 10.3969/j.issn.0253-2409.2020.02.009 [40] FELICZAK-GUZIKA. Hierarchical zeolites: Synthesis and catalytic properties[J]. Microporous Mesoporous Mater,2009,259(15):126−130. [41] HOLM M S, TAARNING E, EGEBLAD K, CHRISTENSEN C H. Catalysis with hierarchical zeolites[J]. Catal Today,2011,168(1):3−16. [42] 高玥, 黄星亮, 字琴, 彭文宇, 张鑫, 田洪锋, 董乐, 刘宗俨. NaOH改性ZSM-5分子筛在苯、甲醇烷基化反应中的应用[J]. 燃料化学学报,2019,47(9):1104−1110. doi: 10.3969/j.issn.0253-2409.2019.09.010GAO Yue, HUANG Xing-liang, ZI Qin, PENG Wen-yu, ZHANG Xin, TIAN Hong-feng, Dong Le, Liu Zong-yan. Application of NaOH modified ZSM-5 molecular sieve in benzene and methanol alkylation[J]. J Fuel Chem Technol,2019,47(9):1104−1110. doi: 10.3969/j.issn.0253-2409.2019.09.010 [43] 孙逊. 多级孔ZSM-5分子筛用于催化甲醇芳构化反应性能研究[D]. 上海: 上海师范大学, 2018.SUN Xun. Multistage pore ZSM-5 molecular sieve for catalytic aromatization of methanol[D]. Shanghai: Shaihai: Normal University, 2018. -





 下载:
下载: