Hydration of α-pinene catalyzed by oxalic acid/polyethylene glycol deep eutectic solvents
-
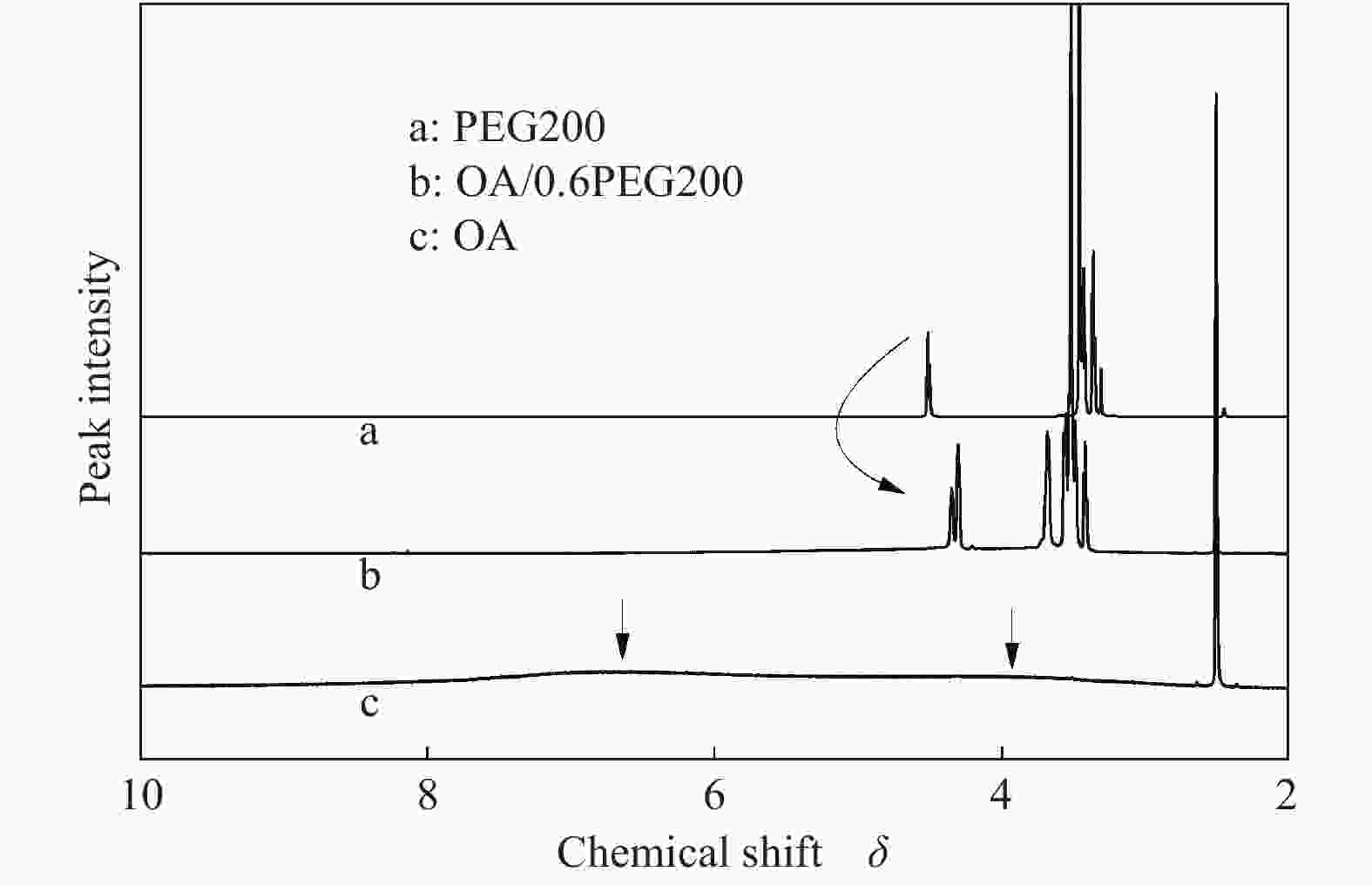
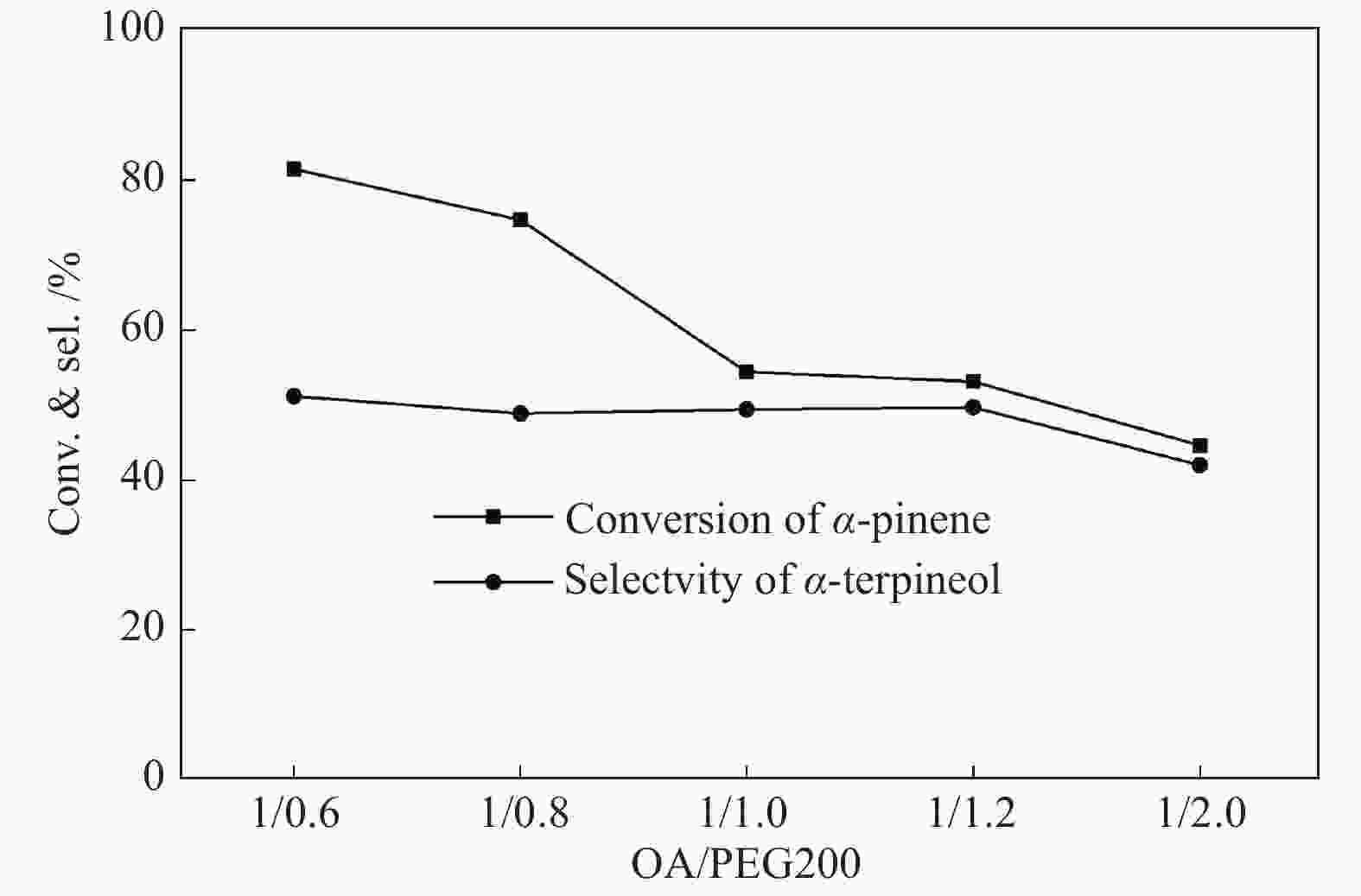
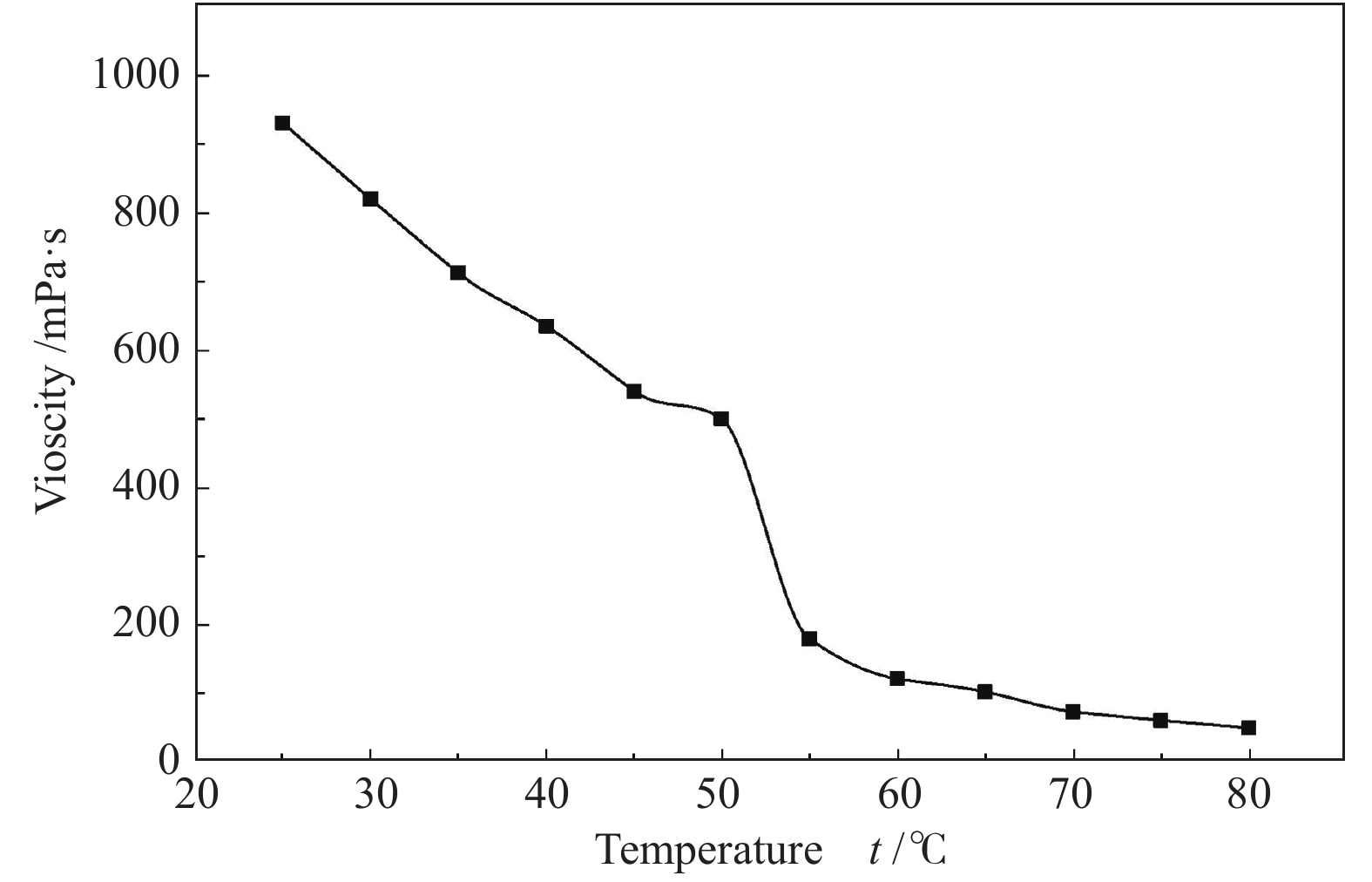
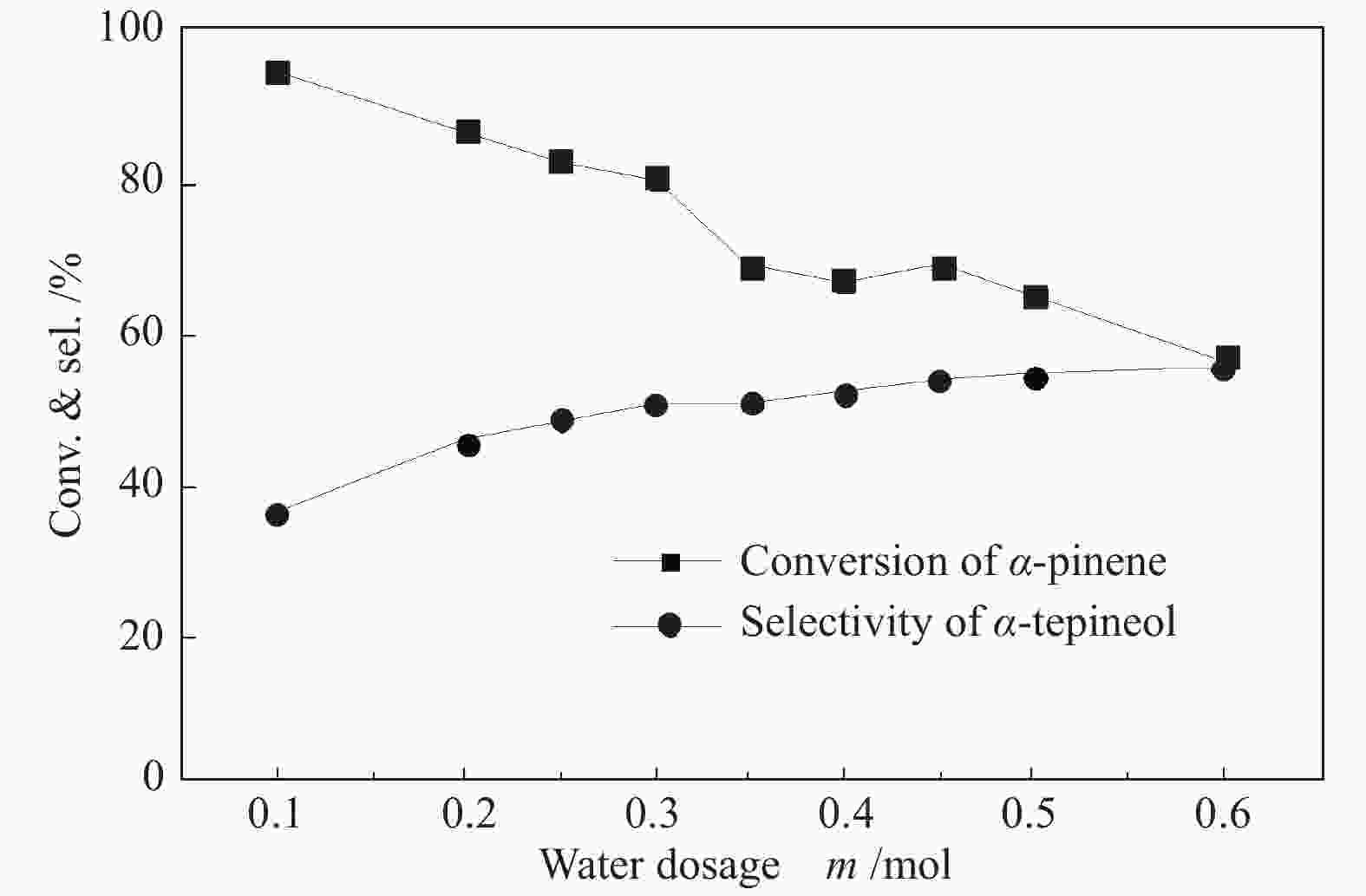
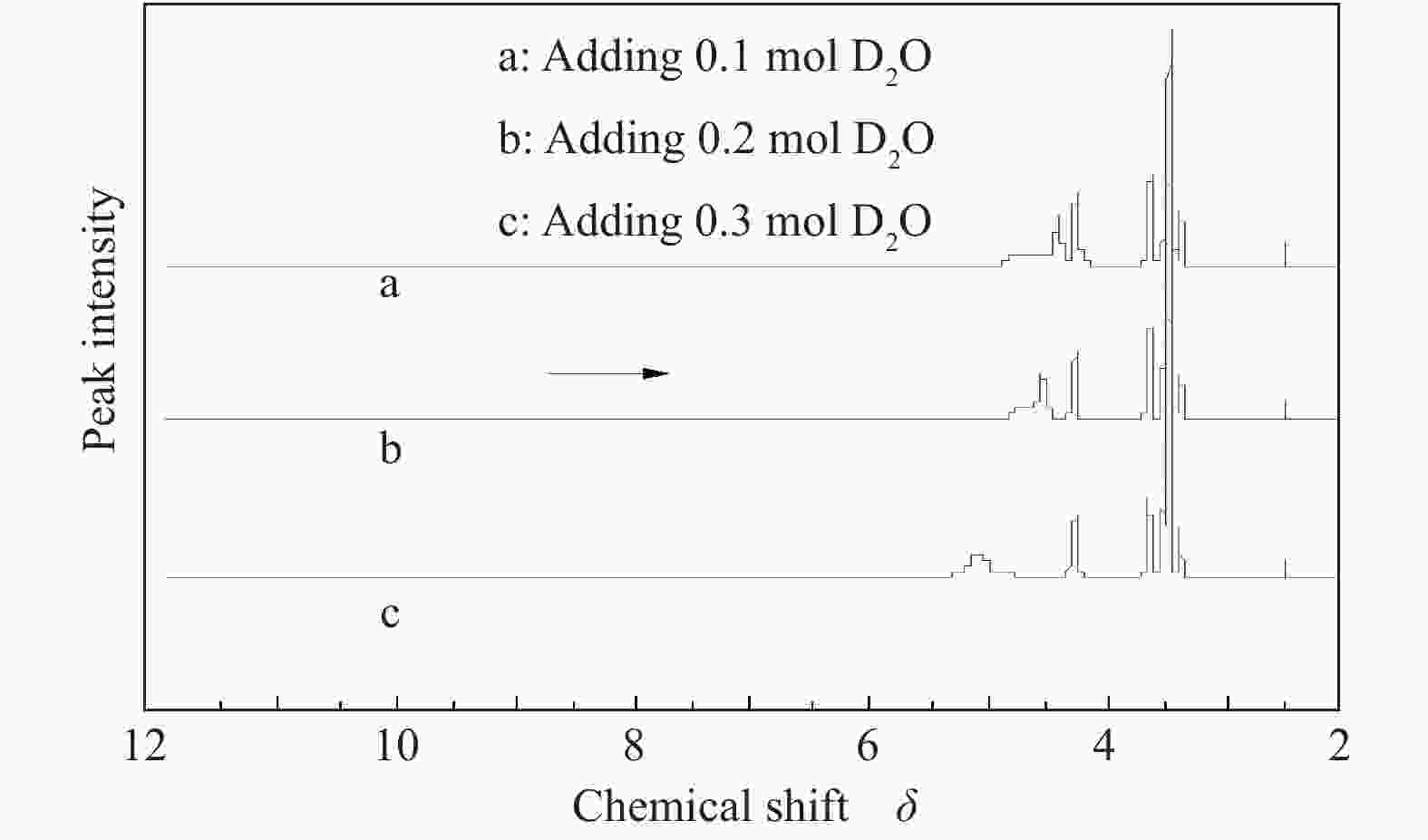
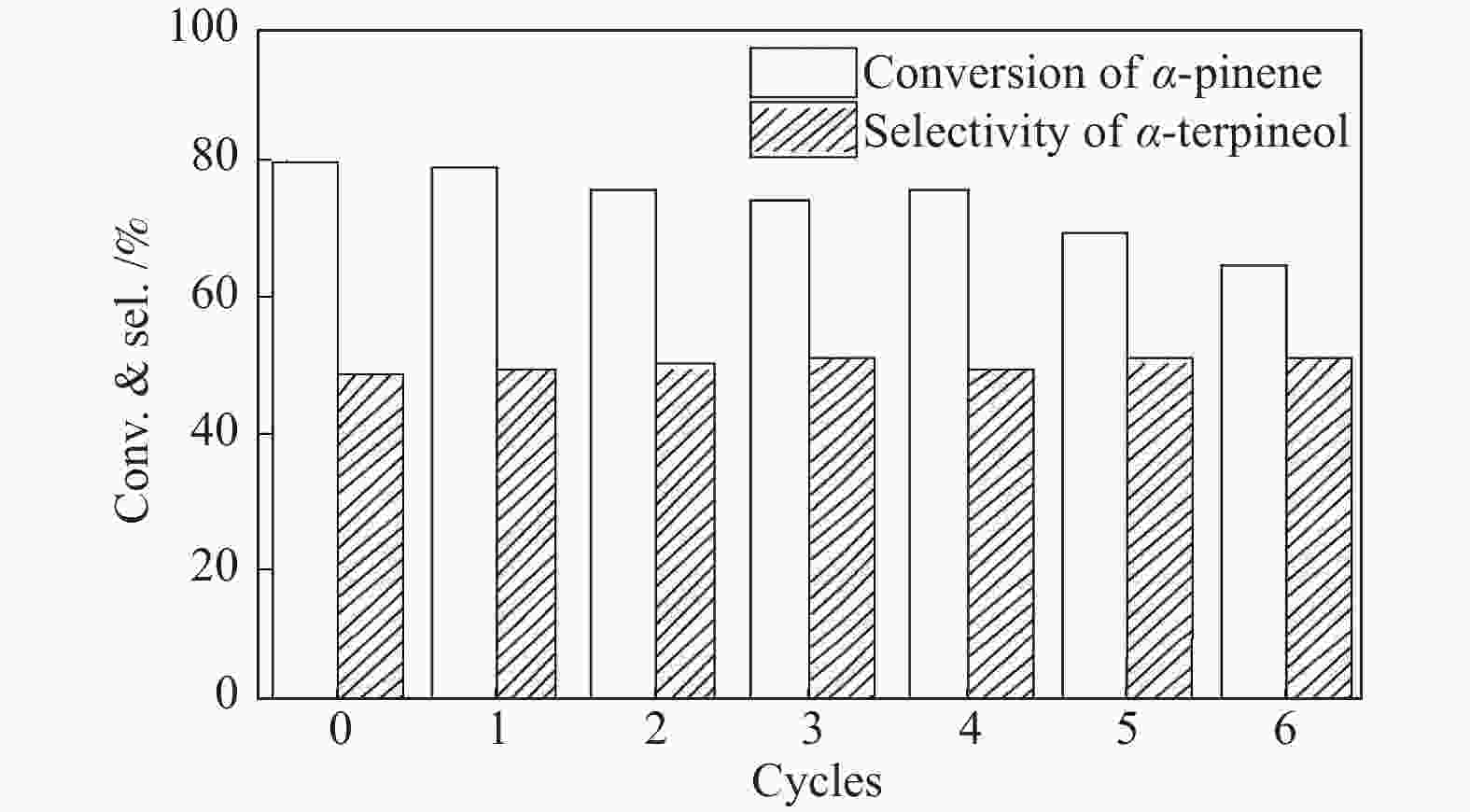
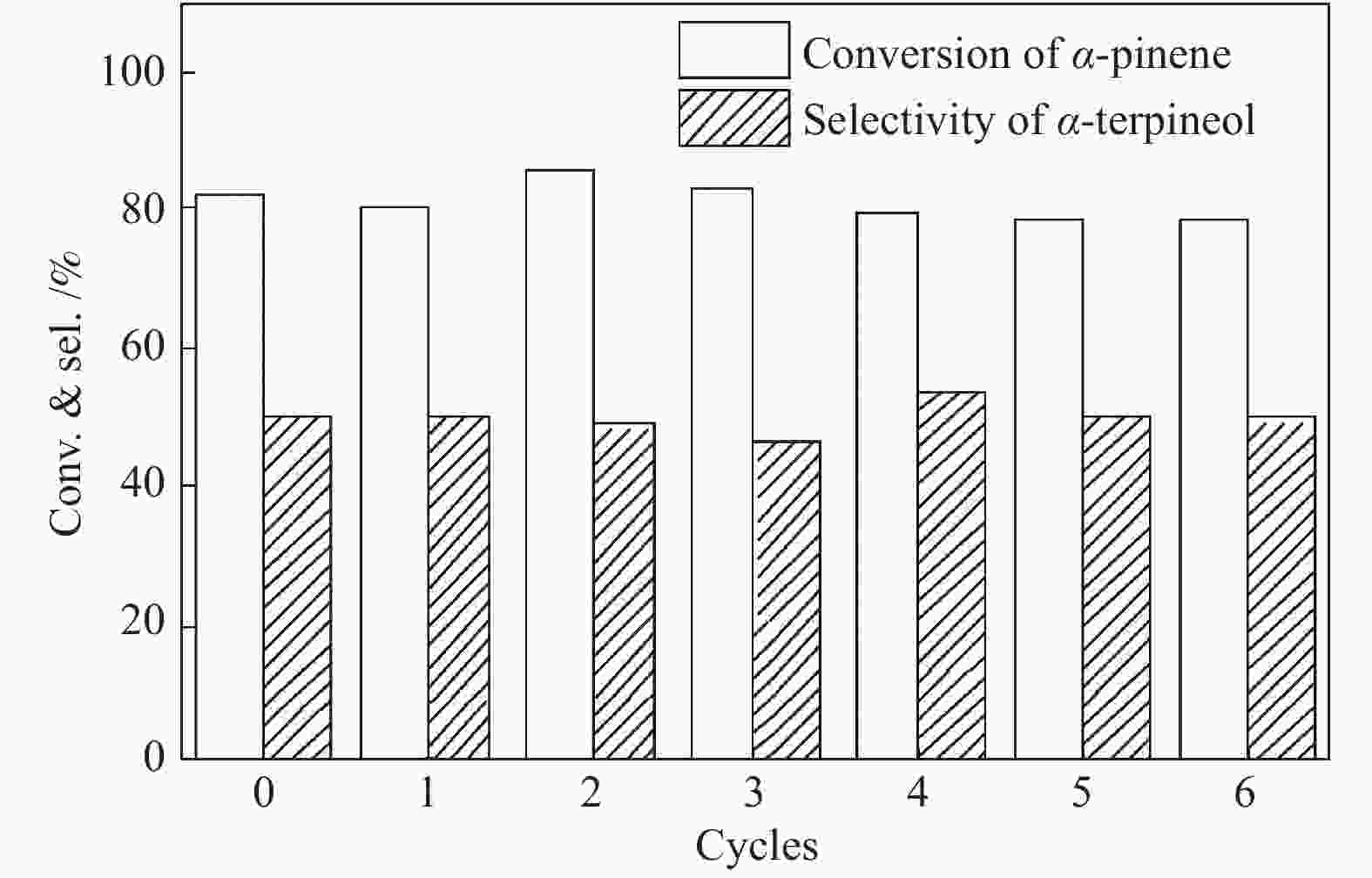
摘要: 以天然有机二元羧酸草酸(OA)作为氢键供体,各种聚合度的聚乙二醇(PEG)作为氢键受体,构建出羧酸功能化低温共熔体(DES),将其用于催化α-蒎烯水合制备α-松油醇的反应中。傅里叶变换红外光谱(FT-IR)、核磁共振氢谱(1H NMR)、热重分析(TGA)等表征证实了OA与PEG之间氢键的形成。DES中PEG组分的存在对其酸强度影响较小,但随PEG分子量和用量的增加,DES总酸量减小,从而降低其催化活性。研究表明,分子量最小的PEG200与OA制备的OA/0.6PEG200 DES具有较佳的催化 α-蒎烯水合反应性能,在DES催化剂用量0.03 mol(以OA计),α-蒎烯用量0.06 mol,水用量0.3 mol,反应温度75 ℃,反应时间8 h的优化条件下,获得81.5%的α-蒎烯转化率及51.2%的α-松油醇选择性。催化剂相反应结束后静置冷藏过夜即可分层分离,且循环使用性能良好。该OA/0.6PEG200 低温共熔体制备简单,原子经济性高,为一步法催化α-松油醇的清洁制备开辟了新路线。Abstract: A series of carboxylic acid-functionalized deep eutectic solvents (DES) were constructed by a natural organic dicarboxylic acid, oxalic acid (OA), as the hydrogen bond donor and the polyethylene glycol (PEG) with different polymerization degrees as the hydrogen bond acceptors, which are used in the hydration of α-pinene to produce α-terpineol. Fourier transform infrared spectroscopy (FT-IR), proton nuclear magnetic resonance spectroscopy (1H NMR), and thermogravimetric analysis (TGA) were used to prove the hydrogen bonding between OA and PEG. It is found that the presence of PEG has a less impact on the acid strength of DES. However, an increase in both molecular weight and dosage of PEG results in a decrease in total acidity and catalytic activity. Among them, OA/0.6PEG200, a DES catalyst prepared by PEG with the smallest molecular weight, exhibits a favorable catalytic performance. Under an optimal reaction condition with 0.03 mol of DES (based on OA), 0.06 mol of α-pinene, 0.3 mol of water, at 75 °C for 8 h, an α-pinene conversion of 81.5% and an α-terpineol selectivity of 51.2% are obtained. The catalyst phase can be separated by refrigerating overnight after reaction and reused directly with relatively stable catalytic performance. Thus, OA/0.6PEG200, as a DES catalyst prepared by a simple and highly atom economical process, will offer a clean catalytic route for the one-step production of α-terpineol.
-
Key words:
- α-pinene /
- α-terpineol /
- hydration /
- deep eutectic solvents
-
表 1 聚乙二醇聚合度对DES催化性能的影响
Table 1 The effect of the degree of polymerization of polyethylene glycol on the catalysis of DES
HBD HBA Conversion of
α-pinene/ %Selectivity of
α-terpineol/%Distribution of reaction products/ % isomerization products e f a b c d OA − 51.5 26.7 4.5 11.6 24.2 26.5 30.4 2.8 OA PEG200 91.7 45.0 4.6 16.4 18.8 6.4 51.0 2.8 OA PEG400 58.6 50.8 7.5 15.1 15.8 5.4 52.3 3.9 OA PEG600 61.4 49.1 7.4 16.1 16.2 4.1 51.1 5.1 OA PEG2000 53.0 50.0 7.8 15.1 16.1 4.2 53.2 3.6 reaction conditions: 0.06 mol of α-pinene, 0.3 mol of water, 0.03 mol (based on OA) of catalyst, 80 ℃, 8 h; products: a (camphene),
b (limonene), c (terpinolene), d (other terpenes), e (total hydration products), f (other by-products)表 2 DES催化剂的酸量
Table 2 Acidity of DES catalyst
Catalyst Acidity/ (mmol H+·g−1) OA 24.07 OA/ 0.6PEG200 6.62 OA/ 0.6PEG400 4.49 OA/ 0.6PEG600 3.71 OA/ 0.6PEG2000 1.09 OA/ 0.8PEG200 5.22 OA/ 1.0PEG200 4.32 OA/ 1.2PEG200 3.67 OA/ 2.0PEG200 1.83 表 3 DES催化剂的密度
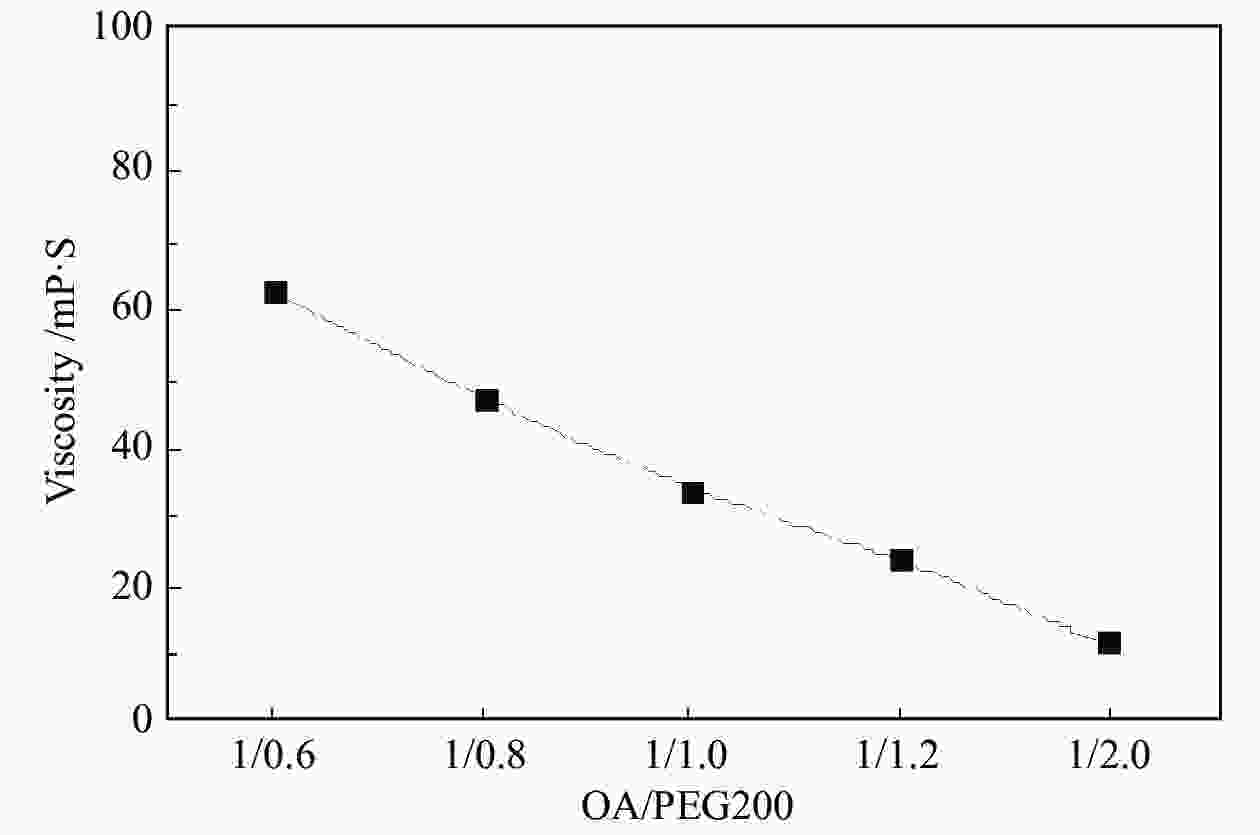
Table 3 Density of DES catalysts
DES ρ/(g·cm−3) OA/ 0.6PEG200 1.303 OA/ 0.8PEG200 1.256 OA/ 1.0PEG200 1.213 OA/ 1.2PEG200 1.190 OA/ 2.0PEG200 1.148 表 4 柠檬烯、异松油烯、α-松油醇的相互转化
Table 4 Mutual conversion of limonene, terpinolene, and α-terpineol
Reactants Components of the reaction mixture/ % isomers ofterpene e f g a b c d Limonene − 87.7 1.3 4.0 5.4 0.5 1.1 Terpinolene − 4.0 76.0 18.0 0.9 0.4 0.7 α-terpineol − 11.0 11.0 9.0 56.5 11.9 0.6 reaction conditions: 0.06 mol of reactants, 0.3 mol of water, 0.03 mol(based on OA) of OA/0.6PEG200, 75 ℃, 8 h; products: a (camphene), b (limonene), c (terpinolene), d (other terpenes), e (α-terpineol), f (other hydration products), g (by-products) -
[1] SARATHY S M, O WALD P, HANSEN N, KOHSE-HÖINGHAUS K. Alcohol combustion chemistry[J]. Prog Energy Combust Sci,2014,44:40−102. [2] VALLINAYAGAM R, VEDHARAJ S, NASER N, ROBERTS W L, DIBBLE R W, SARATHY S M. Terpineol as a novel octane booster for extending the knock limit of gasoline[J]. Fuel,2017,187:9−15. [3] VALLINAYAGAM R, VEDHARAJ S, ROBERTS W L, SARATHY S M, VEDHARAJ S. Performance and emissions of gasoline blended with terpineol as an octane booster[J]. Renewable Energy,2017,101:1087−1093. [4] 赵振东, 刘先章. 松节油的精细化学利用(Ⅱ)—松节油合成日化香料(上)[J]. 生物质化学工程,2001,(2):41−46. doi: 10.3969/j.issn.1673-5854.2001.02.011ZHAO Zhen-dong, LIU Xian-zhang. Fine chemical utilization of turpentine oil (Ⅱ) —synthesis of perfume from turpentine oil (Part I)[J]. Bio Chem Eng,2001,(2):41−46. doi: 10.3969/j.issn.1673-5854.2001.02.011 [5] 赵振东, 刘先章. 松节油的精细化学利用(Ⅶ)—松节油合成功能材料[J]. 生物质化学工程,2002,(1):36−41. doi: 10.3969/j.issn.1673-5854.2002.01.011ZHAO Zhen-dong, LIU Xian-zhang. Fine chemical utilization of turpentine oil (Ⅶ) —Synthesis of functional materials from turpentine oil[J]. Bio Chem Eng,2002,(1):36−41. doi: 10.3969/j.issn.1673-5854.2002.01.011 [6] 王宗德, 宋湛谦. 松节油合成香料的研究现状(一)[J]. 精细与专用化学品,2003,(12):1−3.WANG Zong-de, SONG Zhan-qian. Research status of turpentine synthetic perfume (1)[J]. Fine Spec Chem,2003,(12):1−3. [7] 古研, 赵振东, 毕良武, 李冬梅, 王婧. 马尾松松节油标准样品的定值研究[J]. 生物质化学工程,2011,(1):30−33.GU Yan, ZHAO Zhen-dong, BI Liang-wu, LI Dong-mei, WANG Jing. Determination of masson pine turpentine standard sample[J]. Bio Chem Eng,2011,(1):30−33. [8] 于世涛, 刘福胜, 解从霞, 李露. $ {\rm{SO}}_4^{2 - } $ /SiO2-ZrO2复合固体超强酸催化 α-蒎烯水合反应[J]. 精细化工,2004,21(3):178−180. doi: 10.3321/j.issn:1003-5214.2004.03.006YU Shi-tao, LIU Fu-sheng, XIE Cong-xia, LI Lu.$ {\rm{SO}}_4^{2 - } $ /SiO2 -ZrO2 composite solid super acid catalyzed the hydration reaction of α-pinene[J]. Fine Chem,2004,21(3):178−180. doi: 10.3321/j.issn:1003-5214.2004.03.006[9] VALENTE H, VITAL J. Hydration of α-pinene and camphene over USY zeolites[J]. Stud Surf Sci Catal,1997,108:555−562. [10] 杨高东, 刘勇, 邵玉银, 周政, 张志炳. 松节油直接水合反应研究[J]. 化学工程,2010,38(12):48−52. doi: 10.3969/j.issn.1005-9954.2010.12.012YANG Gao-dong, LIU Yong, SHAO Yu-yin, ZHOU Zheng, ZHANG Zhi-bing. Study on the direct hydration reaction of turpentine[J]. Chem Eng,2010,38(12):48−52. doi: 10.3969/j.issn.1005-9954.2010.12.012 [11] ÁVILA M C, COMELLI N A, RODRÍGUEZ-CASTELLÓN E, JIMÉNEZ-LÓPEZ A, CARRIZO F R, PONZI E N, PONZI M I. Study of solid acid catalysis for the hydration of α-pinene[J]. J Mol Catal A: Chem,2010,322(1 / 2):106−112. [12] COMELLI N A, AVILA M C, VOLZONE C, PONZI M I. Hydration of alpha-pinene catalyzed by acid clays[J]. Cent Eur J Chem,2013,11(5):689−697. [13] 季开慧, 刘仕伟, 于世涛, 刘福胜, 解从霞. 磺烷基咪唑磷酸盐-氯乙酸复合催化体系在 α-蒎烯水合反应中的应用[J]. 林产化学与工业,2007,27(6):77−80. doi: 10.3321/j.issn:0253-2417.2007.06.016JI Kai-hui, LIU Shi-wei, YU Shi-tao, LIU Fu-sheng, XIE Cong-xia. Application of sulfoalkylimidazole phosphate-chloroacetic acid composite catalyst system in α-pinene hydration reaction[J]. Chem Ind For Prod,2007,27(6):77−80. doi: 10.3321/j.issn:0253-2417.2007.06.016 [14] 刘仕伟, 李露, 于世涛, 刘福胜, 宋湛谦. 温控特性的酸功能化离子液体合成及其在 α-蒎烯水合反应中的应用[J]. 催化学报,2011,32(3):96−99.LIU Shi-wei, LI Lu, YU Shi-tao, LIU Fu-sheng, SONG Zhan-qian. Synthesis of acid-functionalized ionic liquid with temperature control characteristics and its application in α-pinene hydration reaction[J]. Chin J Catal,2011,32(3):96−99. [15] YUAN B, ZHONG H, LIU P, LIU X, XIE C, YU F, YU S, ZHANG J. Heteropolyacid bisalt of N-octyl ethoxylated octadecylamine: an efficient and reusable catalyst for carboxylic acid-free hydration of α-pinene[J]. Catal Lett,2016,146(5):929−936. [16] ABBOTT A P, CAPPER G, DAVIES D L, RASHEED R K, TAMBYRAJAH V. Novel solvent properties of choline chloride / urea mixtures[J]. Chem Commun (Camb),2003,(1):70−71. [17] ABBOTT A P, BARRON J C, RYDER K S, WILSON D. Eutectic-based ionic liquids with metal-containing anions and cations[J]. Chem- Eur J,2007,13(22):6495−6501. [18] DAI Y, ROZEMA E, VERPOORTE R, CHOI Y H. Application of natural deep eutectic solvents to the extraction of anthocyanins from Catharanthus roseus with high extractability and stability replacing conventional organic solvents[J]. J Chromatogr A,2016,1434:50−56. [19] XIE X, ZOU X, LU X, CHENG H, XU Q, ZHOU Z, CHANG Y. Electrodeposition of Zn and Cu-Zn alloy from ZnO/CuO precursors in deep eutectic solvent[J]. Appl Surf Sci,2016,385:481−489. [20] HAO L, WANG M, SHAN W, DENG C, REN W, SHI Z, L H. L-proline-based deep eutectic solvents (DESs) for deep catalytic oxidative desulfurization (ODS) of diesel[J]. J Hazard Mater,2017,339:216−222. [21] PHADTARE S B, JARAG K J, SHANKARLING G S. Greener protocol for one pot synthesis of coumarin styryl dyes[J]. Dyes Pigments,2013,97(1):105−112. [22] SONAWANE Y A, PHADTARE S B, BORSE B N, JAGTAP A R, SHANKARLING G S. Synthesis of Diphenylamine-based novel fluorescent styryl colorants by knoevenagel condensation using a conventional method, biocatalyst, and deep eutectic solvent[J]. Org Lett,2018,12(7):1456−1459. [23] CUI Y, CHANG P, YIN J, SHEN M, JIA Y, BAO M. Design, synthesis and properties of acidic deep eutectic solvents based on choline chloride[J]. J Mol Liq,2017,236:338−343. [24] COLMENERO F. Mechanical properties of anhydrous oxalic acid and oxalic acid dihydrate[J]. Phy Chem Chem Phy,2019,21(5):2673−2690. [25] JOSEPH J, JEMMIS E D. Red-, blue-, or no-shift in hydrogen bonds: a unified explanation[J]. J Am Chem Soc,2007,129(15):4620−4632. [26] IJARDAR S P. Deep eutectic solvents composed of tetrabutylammonium bromide and PEG: Density, speed of sound and viscosity as a function of temperature[J]. J Chem Thermodyn,2020,140:105897. [27] JIANG J, YAN C, ZHAO X, LUO H. A PEGylated deep eutectic solvent for controllable solvothermal synthesis of porous NiCo2S4 for efficient oxygen evolution reaction[J]. Green Chem,2017,19(13):3023−3031. [28] ZHAO X, LAN X, YU D, FU H, LIU Z, MU T. Deep eutectic-solvothermal synthesis of nanostructured Fe3S4 for electrochemical N2 fixation under ambient conditions[J]. Chem Commun,2018,54(4):13010−13013. [29] GABRIELE F, CHIARINI M, GERMANI R, TIECCO M. Effect of water addition on choline chloride/glycol deep eutectic solvents: characterization of their structural and physicochemical properties[J]. J Mol Liq,2019,291:111301. -





 下载:
下载: