Oxidation of 5-hydroxylmethylfurfural to 2, 5-furandicarboxylic acid catalyzed by magnetic MnO2-Fe3O4 composite oxides
-
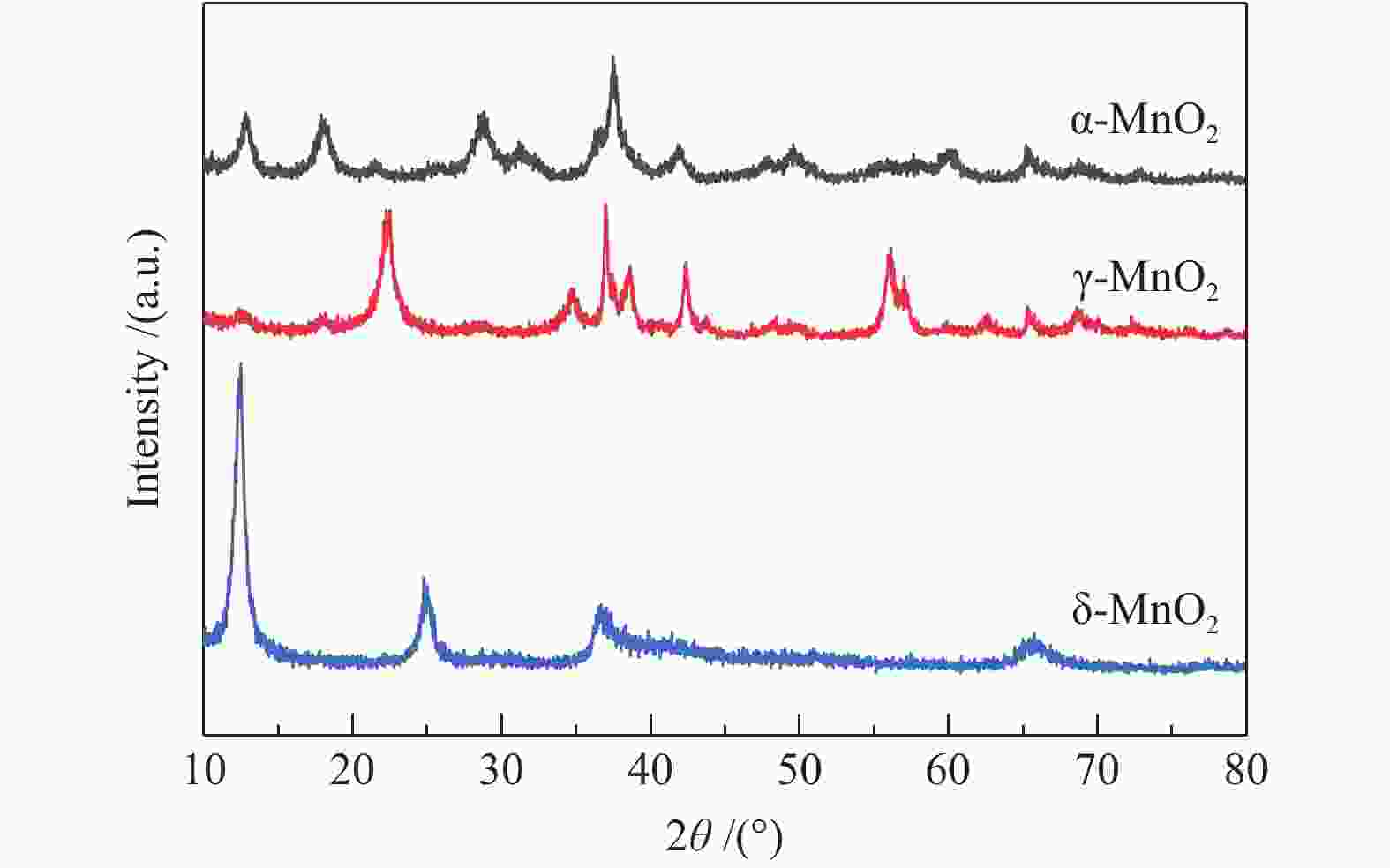
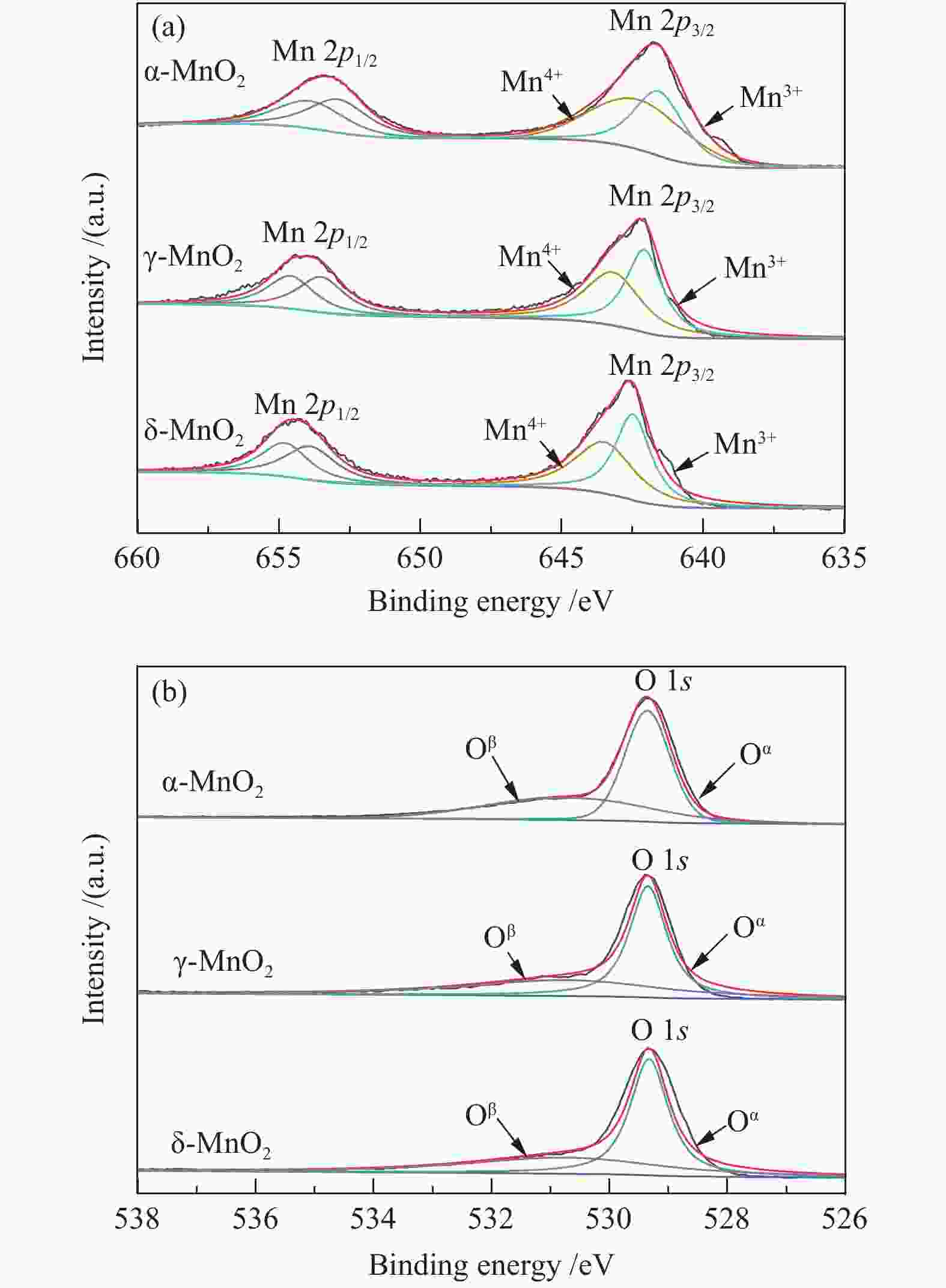
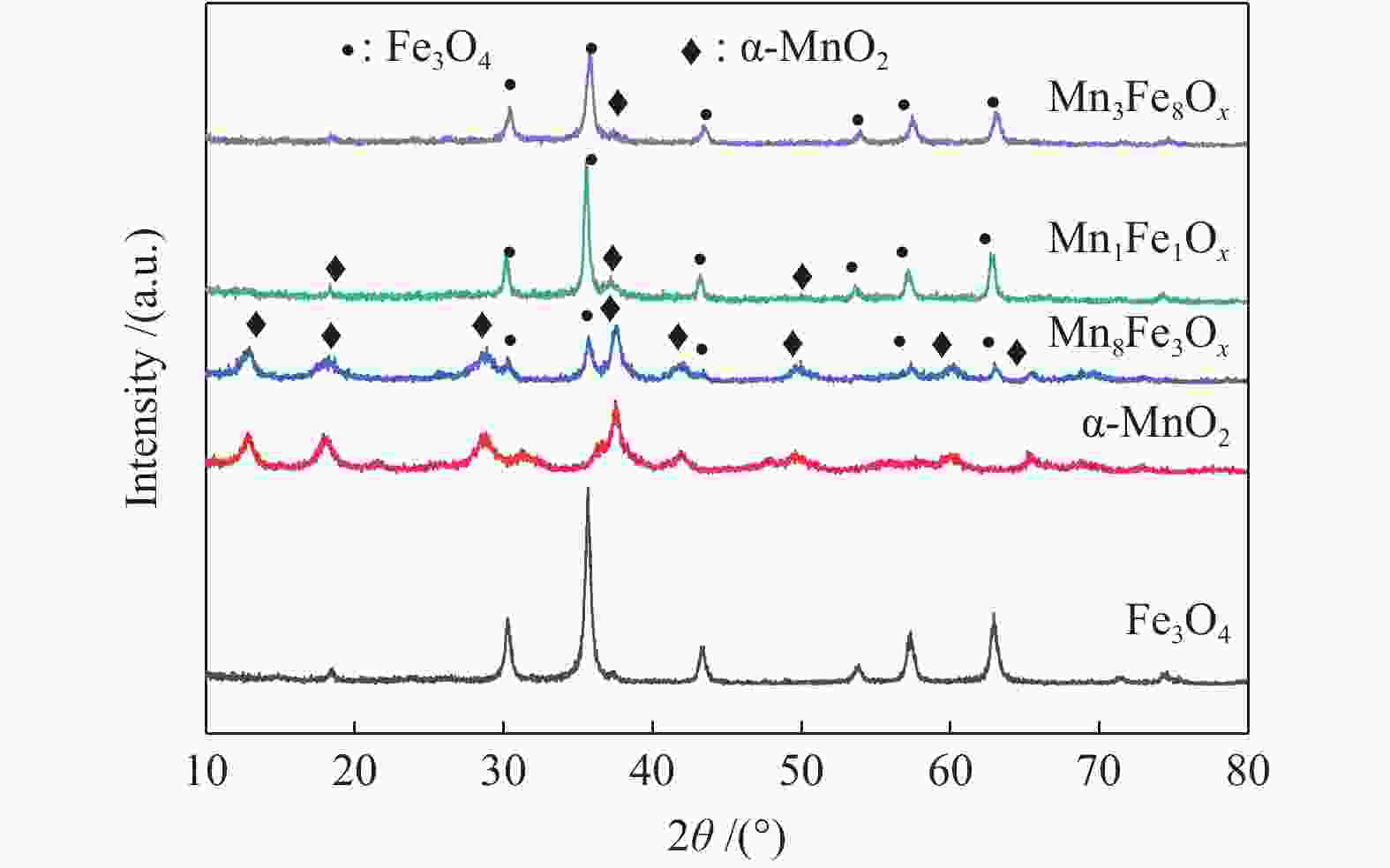
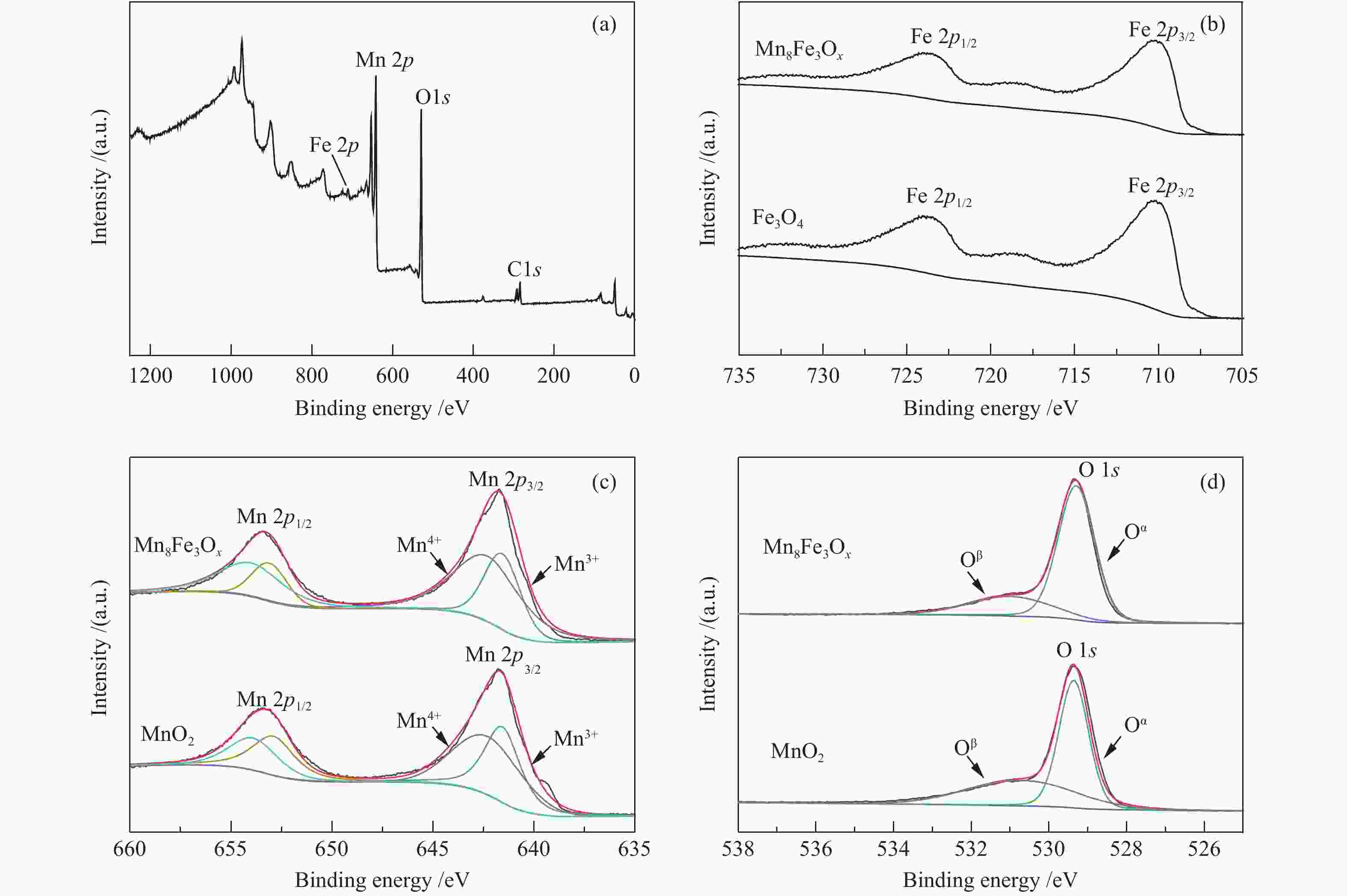
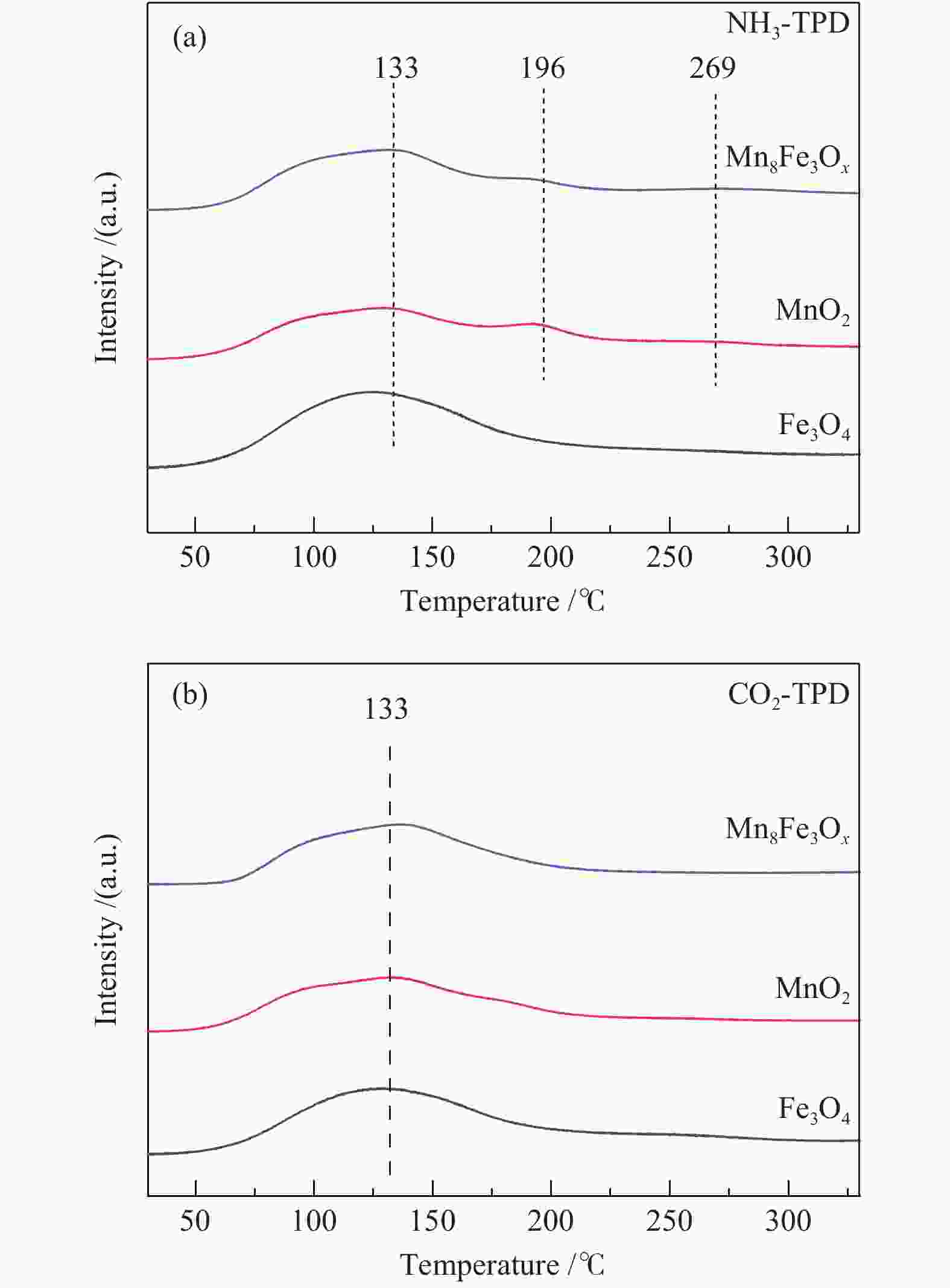
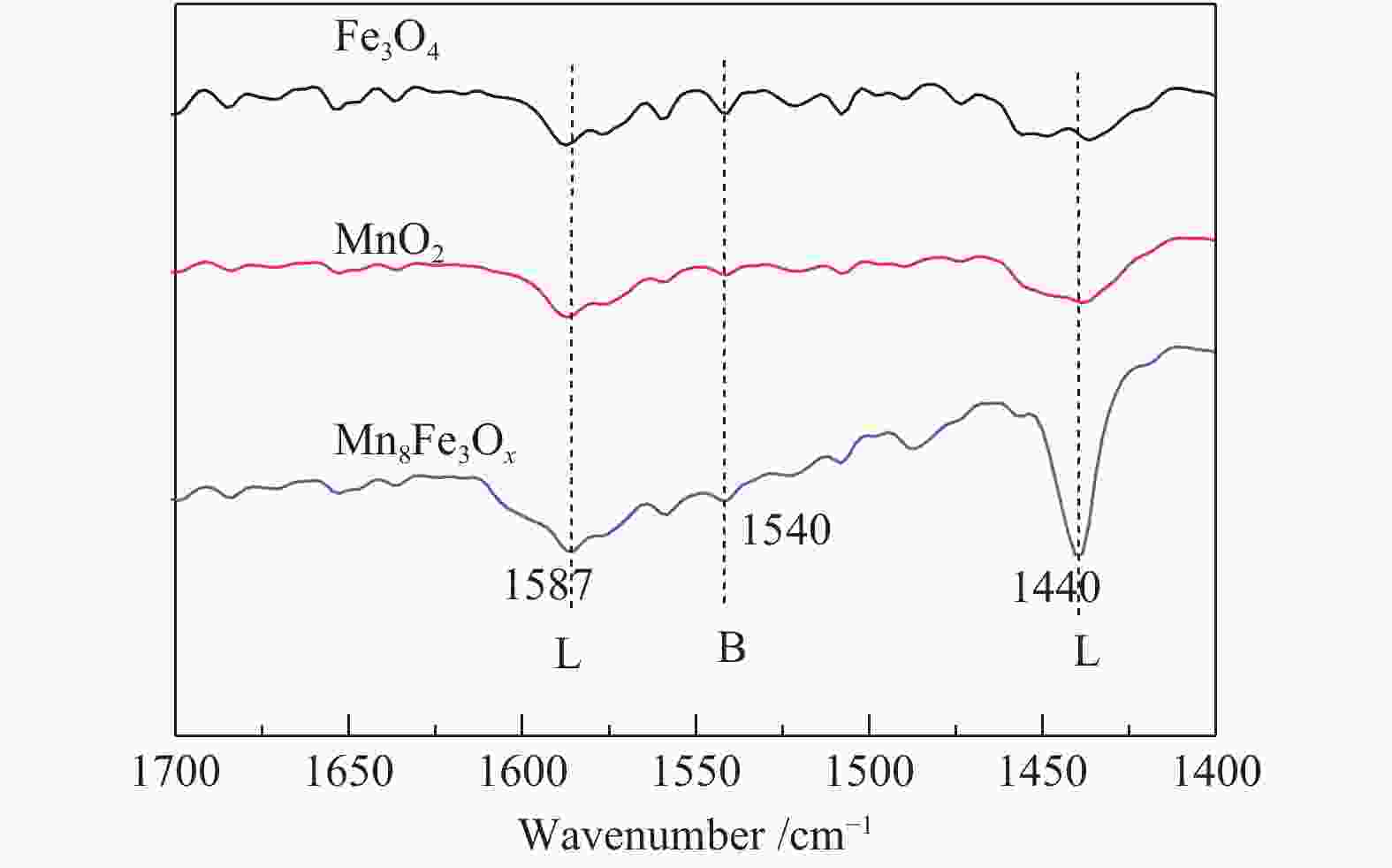
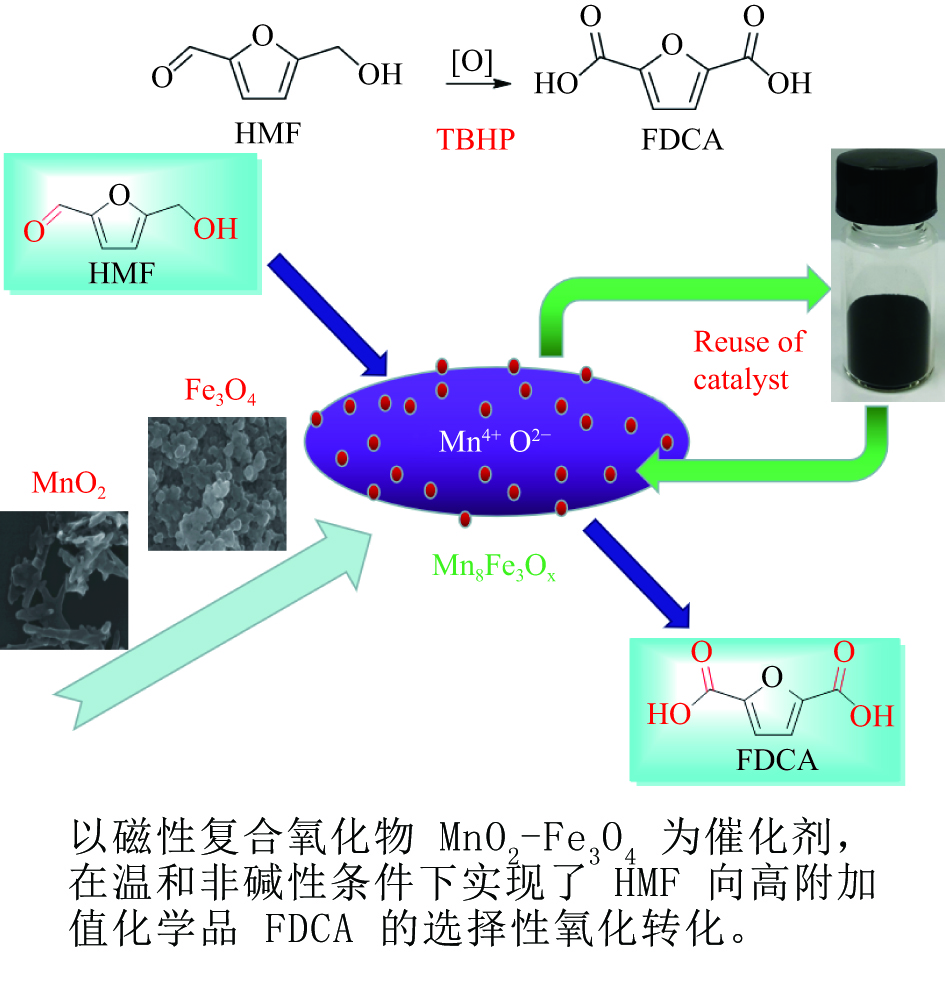
摘要: 以不同晶型的MnO2为催化剂进行5-羟甲基糠醛(HMF)氧化反应,并将催化活性较高的α-MnO2与Fe3O4复合制备磁性MnO2-Fe3O4复合氧化物,采用X射线衍射(XRD)、扫描电镜(SEM)、X射线光电子能谱(XPS)、NH3/CO2程序升温脱附(NH3/CO2-TPD)及吡啶吸附红外光谱(Py-FTIR)对催化剂的结构和性质进行表征和分析。结果表明,复合后的催化剂仍保持α-MnO2和Fe3O4基本结构,但催化剂中活性中心Mn4+·O2−离子对数量增加,使其对HMF氧化反应的催化活性相对α-MnO2和Fe3O4显著提升。对HMF氧化制备2,5-呋喃二甲酸(FDCA)的反应条件进行优化,复合催化剂Mn8Fe3Ox对HMF表现出良好的催化活性,在最优化条件下,HMF可完全转化,FDCA收率为76.9%。
-
关键词:
- 5-羟甲基糠醛 /
- 氧化 /
- 磁性Mn-Fe复合氧化物 /
- 2,5-呋喃二甲酸
Abstract: MnO2 with different crystal structures was used to catalyze the oxidation reaction of 5-hydroxylmethylfurfural (HMF), and α-MnO2 exhibited the highest catalytic activity. Magnetic MnO2-Fe3O4 oxides were prepared by α-MnO2 and Fe3O4 and characterized by X-ray diffraction (XRD), scanning electron microscope (SEM), X-ray photoelectron spectroscopy (XPS), temperature programmed desorption of NH3/CO2 (NH3/CO2-TPD) and Fourier transform infrared reflection spectra of pyridine adsorption (Py-FTIR). The results showed that the composite catalyst still maintained the basic structure of α-MnO2 and Fe3O4, whereas the number of active center Mn4+·O2− ion pair increased compared with α-MnO2 and Fe3O4, which significantly improved the catalytic activity on HMF oxidation reaction. The reaction conditions of HMF oxidation to 2,5-furandicarboxylic acid (FDCA) were optimized. The composite oxide Mn8Fe3Ox showed the best catalytic performance for HMF oxidation. HMF could be completely converted, with 76.9% of FDCA yield under the optimal conditions. -
表 1 不同氧化物催化的HMF氧化反应
Table 1 Perfomance of different catalysts on oxidation of HMF
Catalyst Conversion/% HMFCA
/%DFF
/%FFCA
/%FDCA
/%Total yield/% − 40.5 4.1 10.2 7.8 0 22.1 α-MnO2 83.2 3.0 13.6 19.4 13.3 62.3 γ-MnO2 78.2 2.6 8.6 25.6 4.5 41.3 δ-MnO2 77.3 5.4 7.5 20.3 2.6 35.8 Fe3O4 76.8 2.6 20.1 30.1 5.4 58.2 Mn3Fe8Ox 86.8 3.2 20.1 39.4 14.6 75.3 Mn1Fe1Ox 89.5 4.8 19.8 35.7 15.4 75.7 Mn8Fe3Ox 97.3 5.4 18.7 43.8 23.6 91.5 表 2 催化剂的酸量和减量
Table 2 The acidity and basicity of various catalysts
Sample Acidity/(μmol·g−1) Basicity/(μmol·g−1) Fe3O4 416.0 6.1 MnO2 465.2 11.6 Mn8Fe3Ox 499.5 12.9 表 3 不同溶剂对HMF氧化反应的影响
Table 3 Influence of different solvents on oxidation of HMF
Solvent Conversion
/%HMFCA
/%DFF
/%FFCA
/%FDCA
/%Total yield
/%DMSO 97.3 5.4 18.7 43.8 23.6 91.5 MeCN 81.2 13.5 26.7 9.8 7.8 57.8 DMF 43.5 0.9 0.8 5.1 0 6.8 H2O 28.6 0 13.7 5.4 0 19.1 Ethanol 20.7 5.3 6.9 0.9 0 13.1 表 4 氧化剂和催化剂用量对HMF氧化反应的影响
Table 4 Influence of the dosage of oxidant and catalyst on HMF oxidation
Oxidant dosage
(equiv.)Catalyst dosage
/(g·mL−1)Conversion
/%DFF
/%FFCA
/%FDCA
/%Total yield
/%3.0 0.002 43.6 16.5 19.6 2.1 38.2 6.5 0.002 97.3 18.7 43.8 23.6 91.5 9.0 0.002 100 15.9 35.9 24.4 77.5 6.5 0.010 100 15.4 38.5 37.9 91.8 6.5 0.020 100 10.5 29.5 54.1 94.1 6.5 0.030 100 8.2 28.1 54.4 90.7 6.5 0.040 100 7.3 28.4 54.6 90.3 表 5 正交结果分析
Table 5 Analysis of orthogonal design
Entry Temp./℃ Time/h Oxidant dosage(equiv.) Catalyst dosage/(g·mL−1) FDCA/% 1 70 18 3.0 0.01 13.4 2 70 24 6.5 0.02 68.8 3 70 30 9.0 0.03 75.6 4 80 18 6.5 0.03 40.5 5 80 24 9.0 0.01 74.6 6 80 30 3.0 0.02 27.0 7 90 18 9.0 0.02 37.8 8 90 24 3.0 0.03 10.9 9 90 30 6.5 0.01 32.4 k1 52.6 30.6 17.1 40.1 k2 47.3 51.4 47.2 44.5 k3 27.0 45 62.7 42.3 R 25.6 20.8 45.6 4.4 表 6 Mn8Fe3Ox催化性能与文献报道其他催化剂比较
Table 6 Comparison of the catalytic activity of Mn8Fe3Ox with those of other heterogeneous catalysts reported in the latest literature
Catalyst Oxidants Base Temp./℃ Time/h Conversion/% FDCA/% Ref. Mn8Fe3Ox TBHP − 70 24 100 76.9 this work Au/TiO2 10 bar air 4 equiv. NaOH 130 5 100 84 36 Pd/Al2O3 1 bar O2 1.25 equiv. NaOH 90 8 > 99 78 37 Ru/AC 40 bar air 4 equiv. NaHCO3 100 2 100 75 38 Pt/C 40 bar air 2 equiv. Na2CO3 100 6 99 69 12 Nano-Fe3O4-CoOx TBHP − 80 12 100 68.4 21 Co-Mn-0.25 1 MPa O2 2 equiv. NaHCO3 120 5 99 95 22 1 MPa O2 2 equiv. NaHCO3 90 5 64 ± 3.6 4.6 ± 1.8 MnOx-CeO2 2 MPa O2 4 equiv. KHCO3 110 12 97.9 79.6 31 -
[1] WANG H, ZHU C, LI D, LIU Q, TAN J, WANG C, CAI C, MA L. Recent advances in catalytic conversion of biomass to 5-hydroxymethylfurfural and 2, 5-dimethylfuran[J]. Renewable Sustainable Energy Rev,2019,103:227−247. doi: 10.1016/j.rser.2018.12.010 [2] 王建刚, 张云云, 王勇, 朱丽伟, 崔洪友, 易维明. 分级有序多孔磺化碳催化果糖转化制5-羟甲基糠醛[J]. 燃料化学学报,2016,44(11):1341−1348. doi: 10.3969/j.issn.0253-2409.2016.11.010WANG Jian-gang, ZHANG Yun-yun, WANG Yong, ZHU Li-wei, CUI Hong-you, YI Wei-ming. Catalytic fructose dehydration to 5-hydroxymethylfurfural over sulfonated carbons with hierarchically ordered pores[J]. J Fuel Chem Technol,2016,44(11):1341−1348. doi: 10.3969/j.issn.0253-2409.2016.11.010 [3] KONG X, ZHU Y, FANG Z, KOZINSKI J A, BUTLER I S, XU L, SONG H, WEI X. Catalytic conversion of 5-hydroxymethylfurfural to some value-added derivatives[J]. Green Chem,2018,20(16):3657−3682. doi: 10.1039/C8GC00234G [4] KOMPANETS M O, KUSHCH O V, LITVINOV Y E, PLIEKHOV O L, NOVIKOVA K V, NOVOKHATKO A O, SHENDRIK A N, VASILYEV A V, OPEIDA I O. Oxidation of 5-hydroxymethylfurfural to 2, 5-diformylfuran with molecular oxygen in the presence of N-hydroxyphthalimide[J]. Catal Commun,2014,57:60−63. doi: 10.1016/j.catcom.2014.08.005 [5] 陆强, 廖航涛, 张阳, 张俊姣, 董长青. 果糖低温快速热解制备5-羟甲基糠醛的机理研究[J]. 燃料化学学报,2013,41(9):1070−1076. doi: 10.3969/j.issn.0253-2409.2013.09.007LU Qiang, LIAO Hang-tao, ZHANG Yang, ZHANG Jun-jiao, DONG Chang-qing. Reaction mechanism of low-temperature fast pyrolysis of fructose to produce 5-hydroxymethyl furfural[J]. J Fuel Chem Technol,2013,41(9):1070−1076. doi: 10.3969/j.issn.0253-2409.2013.09.007 [6] ZHANG Z, DENG K. Recent advances in the catalytic synthesis of 2, 5-furandicarboxylic acid and its derivatives[J]. ACS Catal,2015,5(11):6529−6544. doi: 10.1021/acscatal.5b01491 [7] GAO L, DENG K, ZHENG J, LIU B, ZHANG Z. Efficient oxidation of biomass derived 5-hydroxymethylfurfural into 2, 5-furandicarboxylic acid catalyzed by Merrifield resin supported cobalt porphyrin[J]. Chem Eng J,2015,270:444−449. doi: 10.1016/j.cej.2015.02.068 [8] ZUO X, VENKITASUBRAMANIAN P, BUSCH D H, SUBRAMANIAM B. Optimization of Co/Mn/Br-catalyzed oxidation of 5-hydroxymethylfurfural to enhance 2, 5-furandicarboxylic acid yield and minimize substrate burning[J]. ACS Sustainable Chem Eng,2016,4(7):3659−3668. doi: 10.1021/acssuschemeng.6b00174 [9] ZUO X, CHAUDHARI A S, SNAVELY K, NIU F, ZHU H, MARTIN K J, SUBRAMANIAM B. Kinetics of homogeneous 5-hydroxymethylfurfural oxidation to 2, 5-furandicarboxylic acid with Co/Mn/Br catalyst[J]. AIChE J,2017,63(1):162−171. doi: 10.1002/aic.15497 [10] 赖金花, 周硕林, 刘凯, 刘贤响, 尹笃林. 5-羟甲基糠醛选择氧化制2, 5-呋喃二甲酸的研究进展[J]. 精细石油化工,2019,36(2):65−72. doi: 10.3969/j.issn.1003-9384.2019.02.015LAI Jin-hua, ZHOU Shou-lin, LIN Kai, LIU Xian-xiang, YIN Du-lin. Advances on selective oxidation of 5-hydroxymethylfurfura into 2, 5-furandicarboxylic acid[J]. Spec Petrochem,2019,36(2):65−72. doi: 10.3969/j.issn.1003-9384.2019.02.015 [11] DAVIS S E, HOUK L R, TAMARGO E C, DATYE A K, DAVIS R J. Oxidation of 5-hydroxymethylfurfural over supported Pt, Pd and Au catalysts[J]. Catal Today,2011,160(1):55−60. doi: 10.1016/j.cattod.2010.06.004 [12] AIT RASS H, ESSAYEM N, BESSON M. Selective aqueous phase oxidation of 5-hydroxymethylfurfural to 2, 5-furandicarboxylic acid over Pt/C catalysts: influence of the base and effect of bismuth promotion[J]. Green Chem,2013,15(8):2240−2251. doi: 10.1039/c3gc40727f [13] MEI N, LIU B, ZHENG J, LV K, TANG D, ZHANG Z. A novel magnetic palladium catalyst for the mild aerobic oxidation of 5-hydroxymethylfurfural into 2, 5-furandicarboxylic acid in water[J]. Catal Sci Technol,2015,5(6):3194−3202. doi: 10.1039/C4CY01407C [14] TONG X, YU L, CHEN H, ZHUANG X, LIAO S, CUI H. Highly efficient and selective oxidation of 5-hydroxymethylfurfural by molecular oxygen in the presence of Cu-MnO2 catalyst[J]. Catal Commun,2017,90:91−94. doi: 10.1016/j.catcom.2016.11.024 [15] HAYASHI E, KOMANOYA T, KAMATA K, HARA M. Heterogeneously-catalyzed aerobic oxidation of 5-hydroxymethylfurfural to 2, 5-furandicarboxylic acid with MnO2[J]. ChemSusChem,2017,10(4):654−658. doi: 10.1002/cssc.201601443 [16] HAYASHI E, YAMAGUCHI Y, KAMATA K, TSUNODA N, KUMAGAI Y, OBA F, HARA M. Effect of MnO2 crystal structure on aerobic oxidation of 5-hydroxymethylfurfural to 2, 5-furandicarboxylic acid[J]. J Am Chem Soc,2019,141(2):890−900. doi: 10.1021/jacs.8b09917 [17] MA Y, ZHANG T, CHEN L, CHENG H, QI Z. Self-developed fabrication of manganese oxides microtubes with efficient catalytic performance for the selective oxidation of 5-hydroxymethylfurfural[J]. Ind Eng Chem Res,2019,58(29):13122−13132. doi: 10.1021/acs.iecr.9b02650 [18] LIU H, CAO X, WEI J, JIA W, LI M, TANG X, ZENG X, SUN Y, LEI T, LIU S, LIN L. Efficient aerobic oxidation of 5-hydroxymethylfurfural to 2, 5-diformylfuran over Fe2O3-promoted MnO2 catalyst[J]. ACS Sustainable Chem Eng,2019,7(8):7812−7822. doi: 10.1021/acssuschemeng.9b00010 [19] YANG Z Z, DENG J, PAN T, GUO Q X, FU Y. A one-pot approach for conversion of fructose to 2, 5-diformylfuran by combination of Fe3O4-SBA-SO3H and K-OMS-2[J]. Green Chem,2012,14(11):2986−2989. doi: 10.1039/c2gc35947b [20] WANG S, LIU B, YUAN Z, ZHANG Z. Aerobic oxidation of 5-hydroxymethylfurfural into furan compounds over Mo-hydroxyapatite-encapsulated magnetic γ-Fe2O3[J]. J Taiwan Inst Chem Eng,2016,58:92−96. doi: 10.1016/j.jtice.2015.06.002 [21] WANG S, ZHANG Z, LIU B. Catalytic conversion of fructose and 5-hydroxymethylfurfural into 2, 5-furandicarboxylic acid over a recyclable Fe3O4-CoOx magnetite nanocatalyst[J]. ACS Sustainable Chem Eng,2015,3(3):406−412. doi: 10.1021/sc500702q [22] RAO K T V, ROGERS J L, SOUZANCHI S, DESSBESELL L, RAY M B, XU C C. Inexpensive but highly efficient Co-Mn mixed-oxide catalysts for selective oxidation of 5-hydroxymethylfurfural to 2, 5-furandicarboxylic acid[J]. ChemSusChem,2018,11(18):3323−3334. doi: 10.1002/cssc.201800989 [23] LI S, SU K, LI Z, CHENG B. Selective oxidation of 5-hydroxymethylfurfural with H2O2 catalyzed by a molybdenum complex[J]. Green Chem,2016,18(7):2122−2128. doi: 10.1039/C5GC01991E [24] ZHANG Z, LIU B, LV K, SUN J, DENG K. Aerobic oxidation of biomass derived 5-hydroxymethylfurfural into 5-hydroxymethyl-2-furancarboxylic acid catalyzed by a montmorillonite K-10 clay immobilized molybdenum acetylacetonate complex[J]. Green Chem,2014,16(5):2762−2770. doi: 10.1039/c4gc00062e [25] MARTÍNEZ-VARGAS D X, RIVERA DE LA ROSA J, SANDOVAL-RANGEL L, GUZMÁN-MAR J L, GARZA-NAVARRO M A, LUCIO-ORTIZ C J, DE HARO-DEL RÍO D A. 5-Hydroxymethylfurfural catalytic oxidation under mild conditions by Co (Ⅱ), Fe (Ⅲ) and Cu (Ⅱ) Salen complexes supported on SBA-15: Synthesis, characterization and activity[J]. Appl Catal A: Gen,2017,547:132−145. doi: 10.1016/j.apcata.2017.08.035 [26] SAHA B, GUPTA D, ABU-OMAR M M, MODAK A, BHAUMIK A. Porphyrin-based porous organic polymer-supported iron(Ⅲ) catalyst for efficient aerobic oxidation of 5-hydroxymethyl-furfural into 2, 5-furandicarboxylic acid[J]. J Catal,2013,299:316−320. doi: 10.1016/j.jcat.2012.12.024 [27] ZHANG S, SUN X, ZHENG Z, ZHANG L. Nanoscale center-hollowed hexagon MnCo2O4 spinel catalyzed aerobic oxidation of 5-hydroxymethylfurfural to 2, 5-furandicarboxylic acid[J]. Catal Commun,2018,113:19−22. doi: 10.1016/j.catcom.2018.05.004 [28] WEI Z, XIAO S, CHEN M, LU M, LIU Y. Selective oxidation of 5-hydroxymethylfurfural to 2, 5-diformylfuran over a Cu-acetonitrile complex[J]. New J Chem,2019,43(20):7600−7605. doi: 10.1039/C9NJ00465C [29] 谈冠希, 迟姚玲, 李双, 易玉峰, 靳广洲. 锰锆复合氧化物CO催化还原NO性能研究[J]. 燃料化学学报, 2019, 47(10): 1258-1264.TAN Guan-xi, CHI Yao-ling, LI Shuang, YI Yu-feng, JIN Guang-zhou. Performance of manganese-zirconium composite oxide in the catalytic reduction of NO by CO[J]. J Fuel Chem Technol, 47(10): 1258-1264. [30] CHEN L, YANG W, GUI Z, SARAVANAMURUGAN S, RⅡSAGER A, CAO W, QI Z. MnOx/P25 with tuned surface structures of anatase-rutile phase for aerobic oxidation of 5-hydroxymethylfurfural into 2,5-diformylfuran[J]. Catal Today,2019,319:105−112. doi: 10.1016/j.cattod.2018.05.049 [31] HAN X, LI C, LIU X, XIA Q, WANG Y. Selective oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid over MnOx-CeO2 composite catalysts[J]. Green Chem,2017,19:996−1004. doi: 10.1039/C6GC03304K [32] LIU B, ZHANG Z, LV K, DENG K, DUAN H. Efficient aerobic oxidation of biomass-derived 5-hydromethylfurfural to 2,5-diformylfuran catalyzed by magnetic nanoparticle supported manganese oxide[J]. Appl Catal A: Gen,2014,472:64−71. doi: 10.1016/j.apcata.2013.12.014 [33] NEAŢU F, MARIN R, FLOREA M, PETREA N, PAVEL O, PÂRVULESCU V. Selective oxidation of 5-hydroxymethyl furfural over non-precious metal heterogeneous catalysts[J]. Appl Catal B: Environ,2016,180:751−757. doi: 10.1016/j.apcatb.2015.07.043 [34] GAWADE A B, NAKHATE A V, YADAV G D. Selective synthesis of 2, 5-furandicarboxylic acid by oxidation of 5-hydroxymethylfurfural over MnFe2O4 catalyst[J]. Catal Today,2018,309:119−125. doi: 10.1016/j.cattod.2017.08.061 [35] MISHRA D K, CHO J K, KIM Y J. Facile production of 2,5-diformylfuran from base-free oxidation of 5-hydroxymethyl furfural over manganese-cobalt spinels supported ruthenium nanoparticles[J]. J Ind Eng Chem,2018,60:513−519. doi: 10.1016/j.jiec.2017.11.040 [36] CASANOVA O, IBORRA S, CORMA A. Biomass into chemicals: Aerobic oxidation of 5-hydroxymethyl-2-furfural into 2,5-furandicarboxylic acid with gold nanoparticle catalysts[J]. ChemSusChem,2009,2:1138−1144. doi: 10.1002/cssc.200900137 [37] SIYO B, SCHNEIDER M, RADNIK J, POHL M M, LANGER P, STEINFELDT N. Influence of support on the aerobic oxidation of HMF into FDCA over preformed Pd nanoparticle based materials[J]. Appl Catal A: Gen,2014,478:107−116. doi: 10.1016/j.apcata.2014.03.020 [38] KERDI F, RASS H A, PINEL C, BESSON M, PERU G, LEGER B, RIO S, MONFLIER E, PONCHEL A. Evaluation of surface properties and pore structure of carbon on the activity of supported Ru catalysts in the aqueous-phase aerobic oxidation of HMF to FDCA[J]. Appl Catal A: Gen,2015,506:206−219. doi: 10.1016/j.apcata.2015.09.002 -





 下载:
下载: