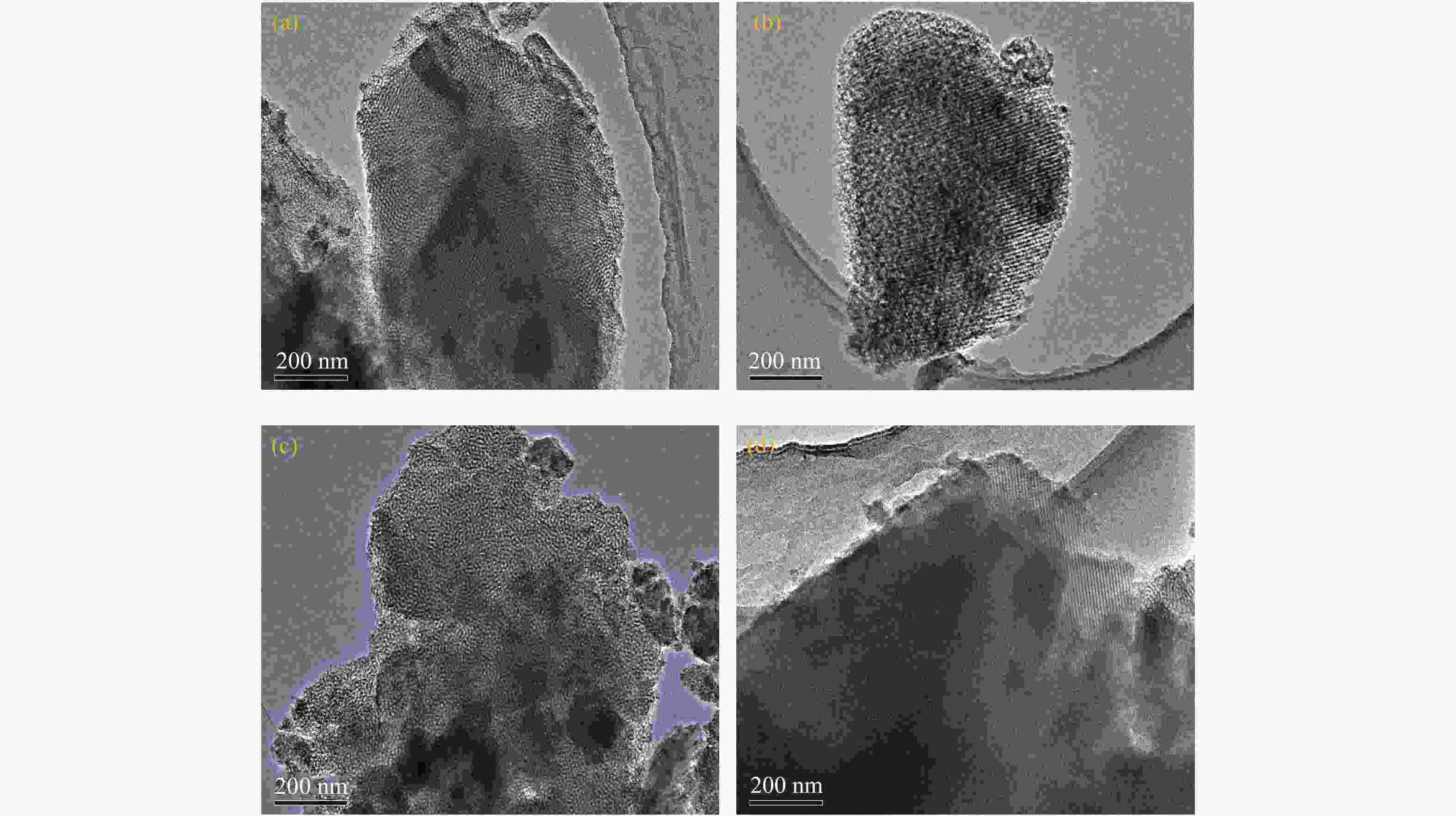
Preparation of CaO/KIT-6 solid base catalyst and its catalytic performance in transesterification
-
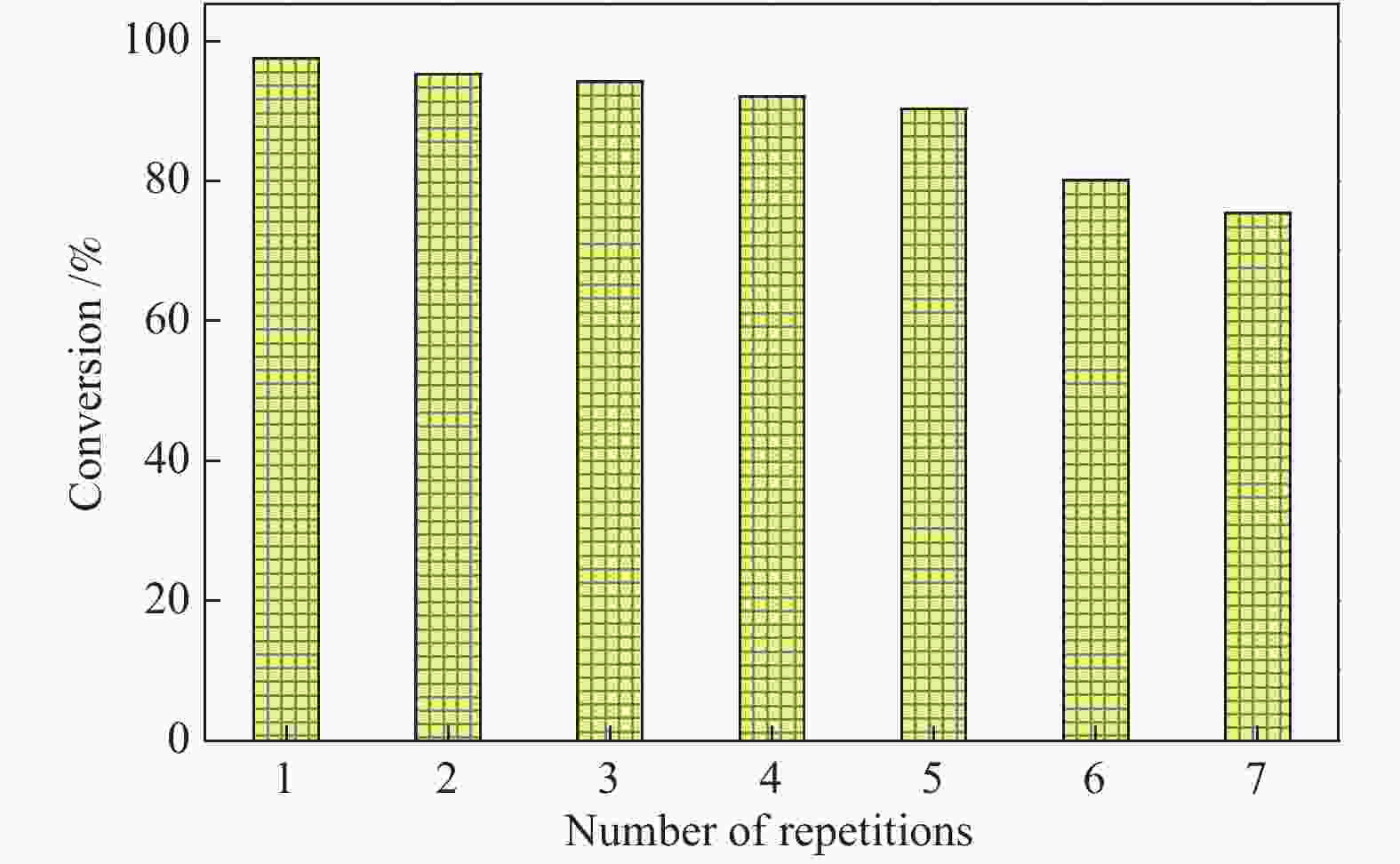
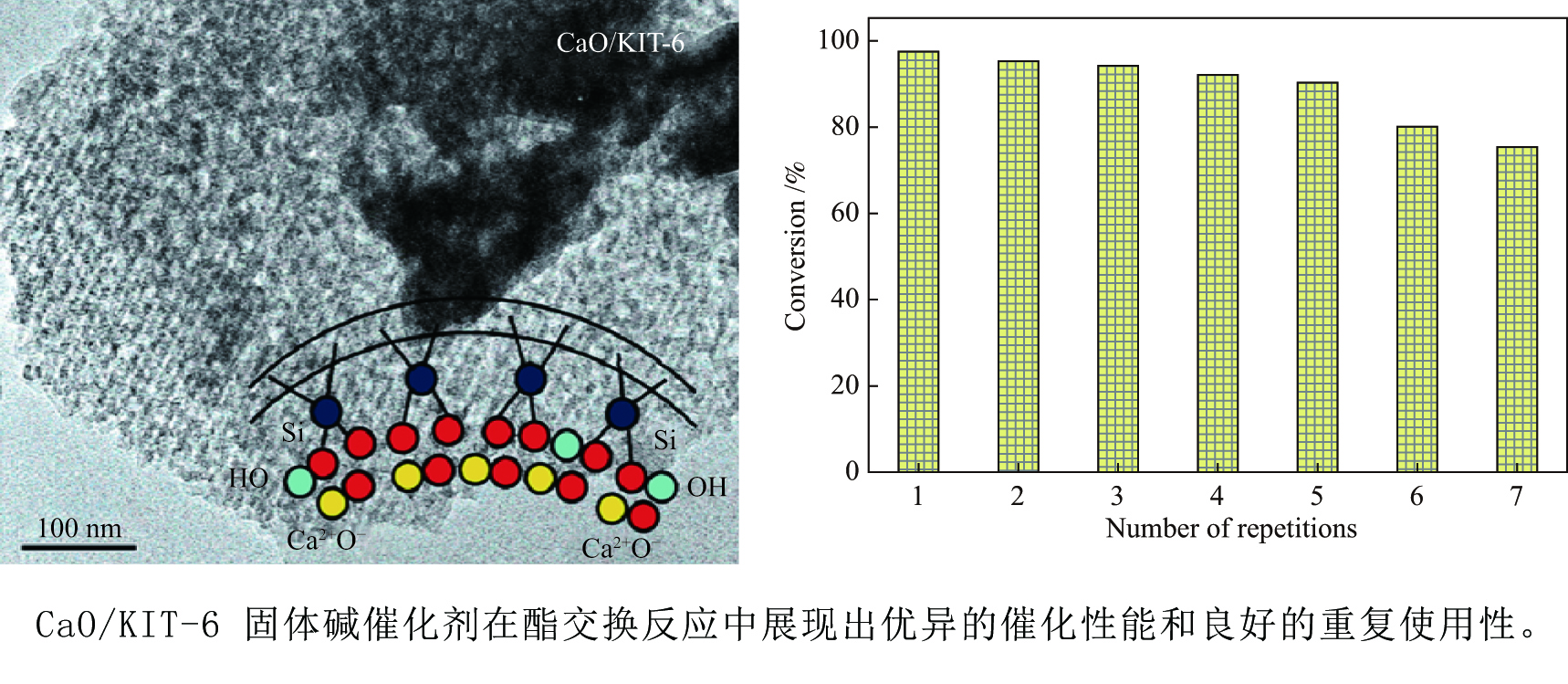
摘要: 采用浸渍法将活性氧化钙颗粒负载在介孔二氧化硅(KIT-6)表面,制备了酯交换反应催化剂CaO/KIT-6,并研究了其在大豆油与甲醇酯交换制备生物柴油反应中的催化性能。通过X射线衍射(XRD)、X射线光电子能谱(XPS)、CO2程序升温脱附(CO2-TPD)等测试手段对催化剂进行表征。在酯交换反应中,当醇油物质的量比为12、反应温度为65 °C、催化剂用量为8%、Ca/Si原子比为0.4、反应时间为2 h时,生物柴油转化率达到99.9%,CaO/KIT-6重复使用五次后催化活性仍保持在90%以上。与CaO及其他负载型催化剂相比,CaO/KIT-6催化剂在较低醇油物质的量之比、较短反应时间展现出更高的催化性能和良好的重复使用性。Abstract: CaO/KIT-6 was successfully prepared by loading activated calcium oxide particles on the surface of mesoporous silica (KIT-6) by post-synthesis method and tested for the transesterification of soybean oil with methanol. The catalyst was characterized by XRD, XPS, CO2-TPD, N2 adsorption-desorption and TEM. It is found that when the molar ratio of methanol to oil is 12, the reaction temperature is 65 °C, the addition of catalyst is 8% and the Ca/Si atomic ratio is 0.4, the conversion of soybean oil exceeds 99.9% at 2 h. After 5 cycles, the catalytic activity decreases slightly and the conversion is still up to 90%. Compared with CaO and other supported catalysts, CaO/KIT-6 catalyst exhibits higher catalytic performance and good reusability at lower methanol-to-oil molar ratio.
-
Key words:
- biodiesel /
- calcium oxide /
- KIT-6 /
- solid base catalyst /
- transesterification
-
图 7 反应参数对CaK-0.4在酯交换反应中的催化活性的影响
Figure 7 Effects of reaction parameters on the catalytic activities of CaK-0.4 for the transesterification reaction: (a): effect of molar ratio of methanol to oil (reaction temperature 65 °C, mass ratio of catalyst to oil 8%, Ca/Si atomic ratio 0.4, reaction time 2 h) (b): effect of reaction time (reaction temperature 65 °C, molar ratio of methanol to oil 12, Ca/Si atomic ratio 0.4, mass ratio of catalyst to oil 8%) (c): effect of Ca/Si atomic ratio (reaction temperature 65 °C, mass ratio of catalyst to oil 8%, molar ratio of methanol to oil 12, reaction time 2 h) (d): effect of mass ratio of catalyst to oil (reaction temperature 65 °C, Ca/Si atomic ratio 0.4, molar ratio of methanol to oil 12, reaction time 2 h)
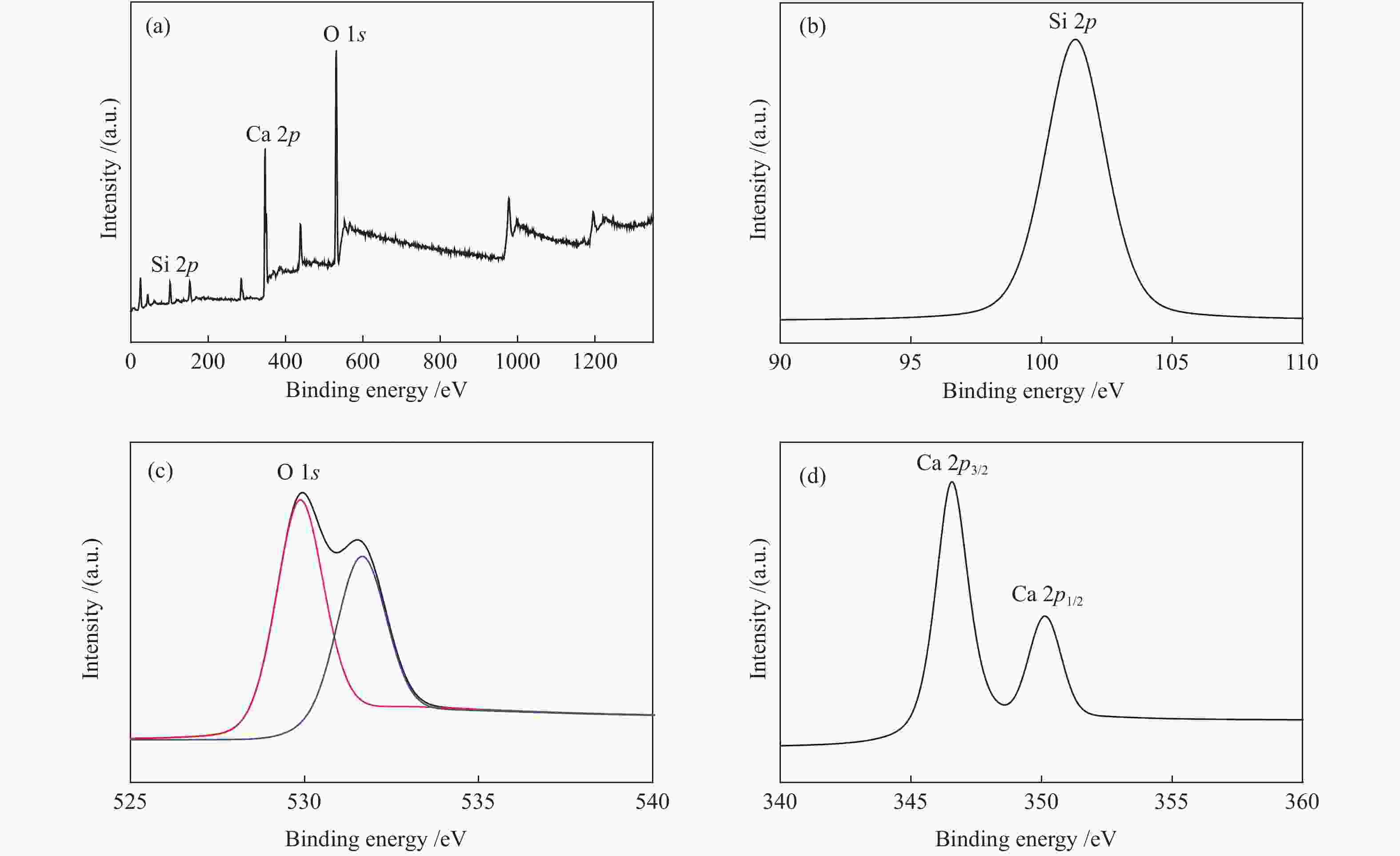
表 1 催化剂的结合能
Table 1 Binding energies of the catalysts
Enerrgy E/eV Si 2p O 1s Ca 2p CaK-0.2 101.9 531.9 530.9 346.8 350.4 CaK-0.4 101.3 529.9 531.6 346.6 350.1 CaK-0.6 100.8 529.9 531.5 346.6 350.1 表 2 催化剂的结构特性
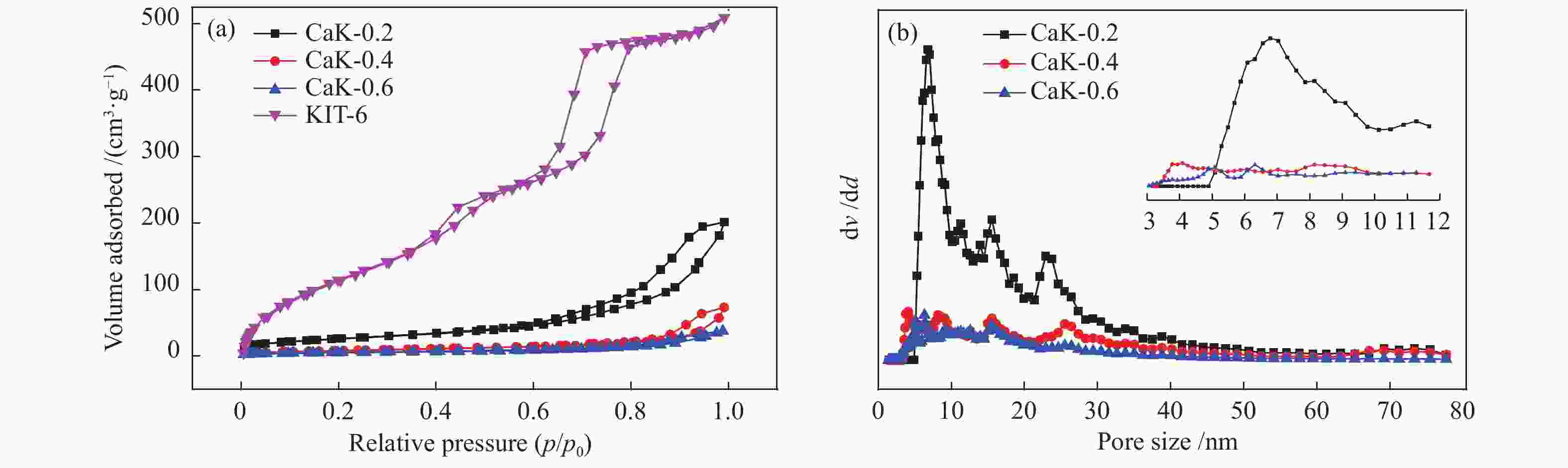
Table 2 Textural properties of the catalysts
Sample SBET/(m2·g−1) vp/(cm3·g−1) dp(av)/nm KIT-6 798 0.74 3.7 CaK-0.2 93 0.28 6.8 CaK-0.4 29 0.10 4.1 CaK-0.6 20 0.10 6.3 CaO 10 0.02 4.9 表 3 氧化钙和负载型催化剂制备生物柴油的催化性能比较
Table 3 Comparison of the activity of calcium oxide and supported catalysts for biodiesel production
Catalyst Feedstock Catalyst characterizations Operation conditions Results Ref. CaO soybeanoil SBET = 0.56 m2·g−1 t = 65 °C, t = 3 h, methanol/
oil = 12:1, catalyst content = 8%FAME yield = 95% [28] CaO sunflower oil Not reported t = 60 °C, t = 100 min, methanol/oil =13:1 conversion = 94% [14] CaO/n-Al2O3 soybean oil SBET = 26 m2·g−1 t = 150 °C, t = 6 h, methanol/oil = 9:1,
Ca loading = 20 mmolmetal/gsupport,
catalyst content = 3%FAME yield = 90% [29] CaO/SBA-15 sunflower oil SBET = 7.4 m2·g−1,vtotal = 0.019 cm3·g−1 t = 60 °C, t = 5 h, methanol/
oil = 12:1, CaO content = 14%,
catalyst content = 1%conversion = 95% [18] CaO/KIT-6 soybean oil SBET = 29.4 m2·g−1,vtotal = 0.098 cm3·g−1 t = 65 °C, t = 2 h, methanol/
oil = 12:1, Ca/Si atomic ratio= 0.4,
catalyst content = 8%conversion = 99.9% present work -
[1] MARINKOVIC D M, STANKOVIC M, VELICKOVIC A V, AVRAMOVIC J M, MILADINOVIC M R, STANKOVIC O O, VELJKOVIC V B, JOVANOVIC D M. Calcium oxide as a promising heterogeneous catalyst for biodiesel production: Current state and perspectives[J]. Renewable Sustainable Energy Rev,2016,56:1387−1408. doi: 10.1016/j.rser.2015.12.007 [2] KIM H J, KANG B S, KIM M J, PARK Y M, KIM D K, LEE J S, LEE K Y. Transesterification of vegetable oil to biodiesel using heterogeneous base catalyst[J]. Catal Today,2004,9395:315−320. [3] EBIURA T, ECHIZEN T, ISHIKAWA A, MURAI K, BABA T. Selective transesterification of triolein with methanol to methyl oleate and glycerol using alumina loaded with alkali metal salt as a solid-base catalyst[J]. Appl Catal A: Gen,2005,283(12):111−116. [4] DORADO M P, BALLESTEROS E, LOPEZ, FRANCISCO J, MITTELBACH M. Optimization of alkali-catalyzed transesterification of brassica carinata oil for biodiesel production[J]. Energy Fuels,2004,18(1):77−83. doi: 10.1021/ef0340110 [5] VICENTE G, MARTINEZ M, ARACIL J. Integrated biodiesel production: a comparison of different homogeneous catalysts systems[J]. Bioresour Technol,2004,92(3):297−305. doi: 10.1016/j.biortech.2003.08.014 [6] LOPEZ D E, GOODWIN J G, BRUCE D A, LOTERO E. Transesterification of triacetin with methanol on solid acid and base catalysts[J]. Appl Catal A: Gen,2005,295(2):97−105. doi: 10.1016/j.apcata.2005.07.055 [7] DEMIRBAS A. Comparison of transesterification methods for production of biodiesel from vegetable oils and fats[J]. Energy Convers Manage,2008,49(1):125−130. doi: 10.1016/j.enconman.2007.05.002 [8] DI SERIO M, MALLARDO S, CAROTENUTO G, TESSER R, SANTACESARIA E. Mg/Al hydrotalcite catalyst for biodiesel production in continuous packed bed reactors[J]. Catal Today,2012,195(1):54−58. doi: 10.1016/j.cattod.2012.01.013 [9] REN Y, HE B, YAN F, WANG H, CHENG Y, LIN L, FENG Y, LI J. Continuous biodiesel production in a fixed bed reactor packed with anion-exchange resin as heterogeneous catalyst[J]. Bioresour Technol,2012,113:19−22. doi: 10.1016/j.biortech.2011.10.103 [10] TANG Y, XU J, ZHANG J, LU Y. Biodiesel production from vegetable oil by using modified CaO as solid basic catalysts[J]. J Clean Prod,2013,42:198−203. doi: 10.1016/j.jclepro.2012.11.001 [11] WU H, ZHANG J, LIU Y, ZHENG J, WEI Q. Biodiesel production from Jatropha oil using mesoporous molecular sieves supporting K2SiO3 as catalysts for transesterification[J]. Fuel Process Technol,2014,119:114−120. doi: 10.1016/j.fuproc.2013.10.021 [12] GRYGLEWICZ S. Rapeseed oil methyl esters preparation using heterogeneous catalysts[J]. Bioresour Technol,1999,70(3):249−253. doi: 10.1016/S0960-8524(99)00042-5 [13] YOOSUK B, UDOMSAP P, PUTTASAWAT B, KRASAE P. Improving transesterification acitvity of CaO with hydration technique[J]. Bioresour Technol,2010,101(10):3784−3786. doi: 10.1016/j.biortech.2009.12.114 [14] GRANNADOS M L, POVES M D Z, ALONSO D M, MARISCAL R, GALISTEO F C, MORENOTOST R, SANTAMARIA J, FIERRO J L G. Biodiesel from sunflower oil by using activated calcium oxide[J]. Appl Catal B: Environ,2007,73(34):317−326. [15] GRANADOS M L, ALONSO D M, ALBARUBIO A C, MARISCAL R, OJEDA M, BRETTES P. Transesterification of Triglycerides by CaO: Increase of the Reaction Rate by Biodiesel Addition[J]. Energy Fuels,2009,23(4):2259−2263. doi: 10.1021/ef800983m [16] 孙辉. 固体碱催化植物油制备生物柴油的基础研究[D]. 杭州: 浙江大学, 2012.SUN Hui. Basic research on the preparation of biodiesel from vegetable oil catalyzed by solid base[D]. Hangzhou: Zhejiang University, 2012. [17] SEMWAL S, ARORA A K, BADONI R P, TULI D K. Biodiesel production using heterogeneous catalysts[J]. Bioresour Technol,2011,102(3):2151−2161. doi: 10.1016/j.biortech.2010.10.080 [18] ALBUQUERQUE M C G, JIMENEZURBISTONDO I, SANTAMARIAGONZALEZ J, MERIDAROBLES J, MORENOTOST R, RODRIGUEZCASTELLON E, JIMENEZLOPEZ A, AZEVEDO D C S, CAVALCANTE C L, MAIRELESTORRES P. CaO supported on mesoporous silicas as basic catalysts for transesterification reactions[J]. Appl Catal A: Gen,2008,334(1):35−43. [19] WU H, ZHANG J, WEI Q, ZHENG J, ZHANG J. Transesterification of soybean oil to biodiesel using zeolite supported CaO as strong base catalysts[J]. Fuel Process Technol,2013,109:13−18. doi: 10.1016/j.fuproc.2012.09.032 [20] KLEITZ F, CHOI S H, RYOO R. Cubic Ia3d large mesoporous silica: synthesis and replication to platinum nanowires, carbon nanorods and carbon nanotubes[J]. Chem Commun,2003,9(17):2136−2137. [21] SONI K, RANA B S, SINHA A K, BHAUMIK A, NANDI M, KUMAR M, DHAR G M. 3-D ordered mesoporous KIT-6 support for effective hydrodesulfurization catalysts[J]. Appl Catal B: Environ,2009,90(1):55−63. [22] KIM T, KLEITZ F, PAUL B, RYOO R. MCM-48-like large mesoporous silicas with tailored pore structure: facile synthesis domain in a ternary triblock copolymer-butanol-water system[J]. J Am Chem Soc,2005,127(20):7601−7610. doi: 10.1021/ja042601m [23] PINHO G P, NEVES A A, QUEIROZ M E L R, SILVERIO F O. Matrix effect in pesticide quantification by gas chromatography[J]. Quim Nova,2009,(32):987−995. [24] THOMMES M, KANEKO K, NEIMARK A V, OLIVIER J P, RODRIGUEZREINOSO F, ROUQUEROL J, SING K S W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report)[J]. Pure Appl Chem,2015,87(9/10):1051−1069. doi: 10.1515/pac-2014-1117 [25] LI H, WANG Y, MA X, WU Z, CUI P, LU W, LIU F, CHU H, WANG Y. A novel magnetic CaO-based catalyst synthesis and characterization: Enhancing the catalytic activity and stability of CaO for biodiesel production[J]. Chem Eng J,2020,391:123549. doi: 10.1016/j.cej.2019.123549 [26] XIE W, WANG H. Immobilized polymeric sulfonated ionic liquid on core-shell structured Fe3O4/SiO2 composites: A magnetically recyclable catalyst for simultaneous transesterification and esterifications of low-cost oils to biodiesel[J]. Renewable Sustainable Energy,2020,145:1709−1719. doi: 10.1016/j.renene.2019.07.092 [27] MEHER L C, VIDYA SAGAR D, NAIK S N. Technical aspects of biodiesel production by transesterification—a review[J]. Renewable Sustainable Energy Rev,2006,10(3):248−268. doi: 10.1016/j.rser.2004.09.002 [28] RUBIOCABALLERO J M, SANTAMARIAGONZALEZ J, MERIDAROBLES J, MORENOTOST R, JIMENEZLOPEZ A, MAIRELESTORRES P. Calcium zincate as precursor of active catalysts for biodiesel production under mild conditions[J]. Appl Catal B: Environ,2009,91(1):339−346. [29] PASUPULETY N, GUNDA K, LIU Y, REMPEL G L, NG F T T. Production of biodiesel from soybean oil on CaO/Al2O3 solid base catalysts[J]. Appl Catal A: Gen,2013,452:189−202. doi: 10.1016/j.apcata.2012.10.006 -





 下载:
下载: