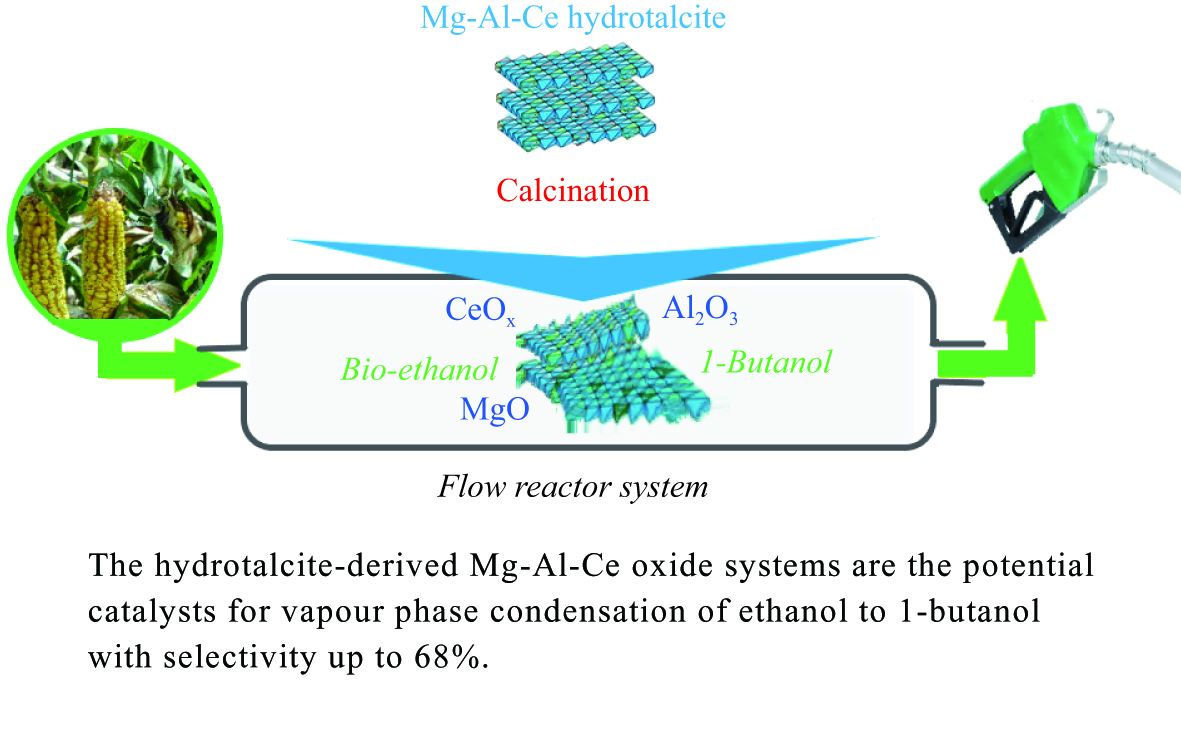
Catalytic performance of ternary Mg-Al-Ce oxides for ethanol conversion into 1-butanol in a flow reactor
-
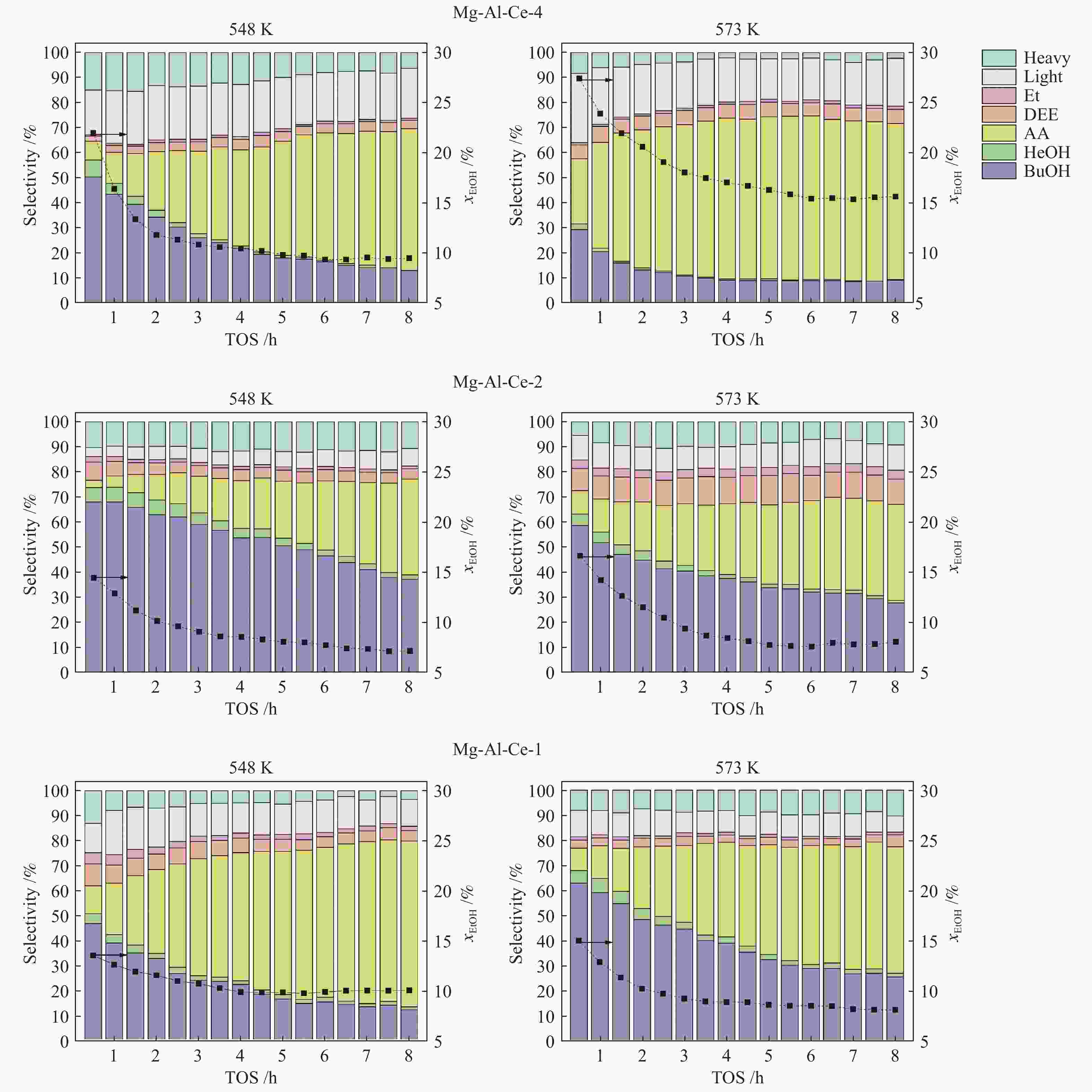
Abstract: An investigation of hydrotalcite-derived ternary Mg-Al-Ce oxides as catalysts for vapour phase condensation of ethanol to 1-butanol in a flow reactor under atmospheric pressure was carried out. The Mg-Al-Ce oxide systems with Mg/(Al + Ce) ratio from 1 to 4 were synthesized and characterized by XRD, SEM, NMR, and XPS. The study of acid-base characteristics of the systems with different Mg/(Al+Ce) ratio by NH3/CO2 quasi-equilibrium thermal desorption techniques shows that the ratio of the catalyst oxide components (Mg, Al, Ce) can provide acid/base capacity ratio close to 3 for the effectivity of the target process. The highest selectivity 68% is reached over Mg-Al-Ce oxide catalyst with the ratio of Mg/(Al+Ce) = 2.
-
Key words:
- ethanol /
- 1-butanol /
- Mg-Al-Ce hydrotalcite-derived oxides /
- acid-base properties
-
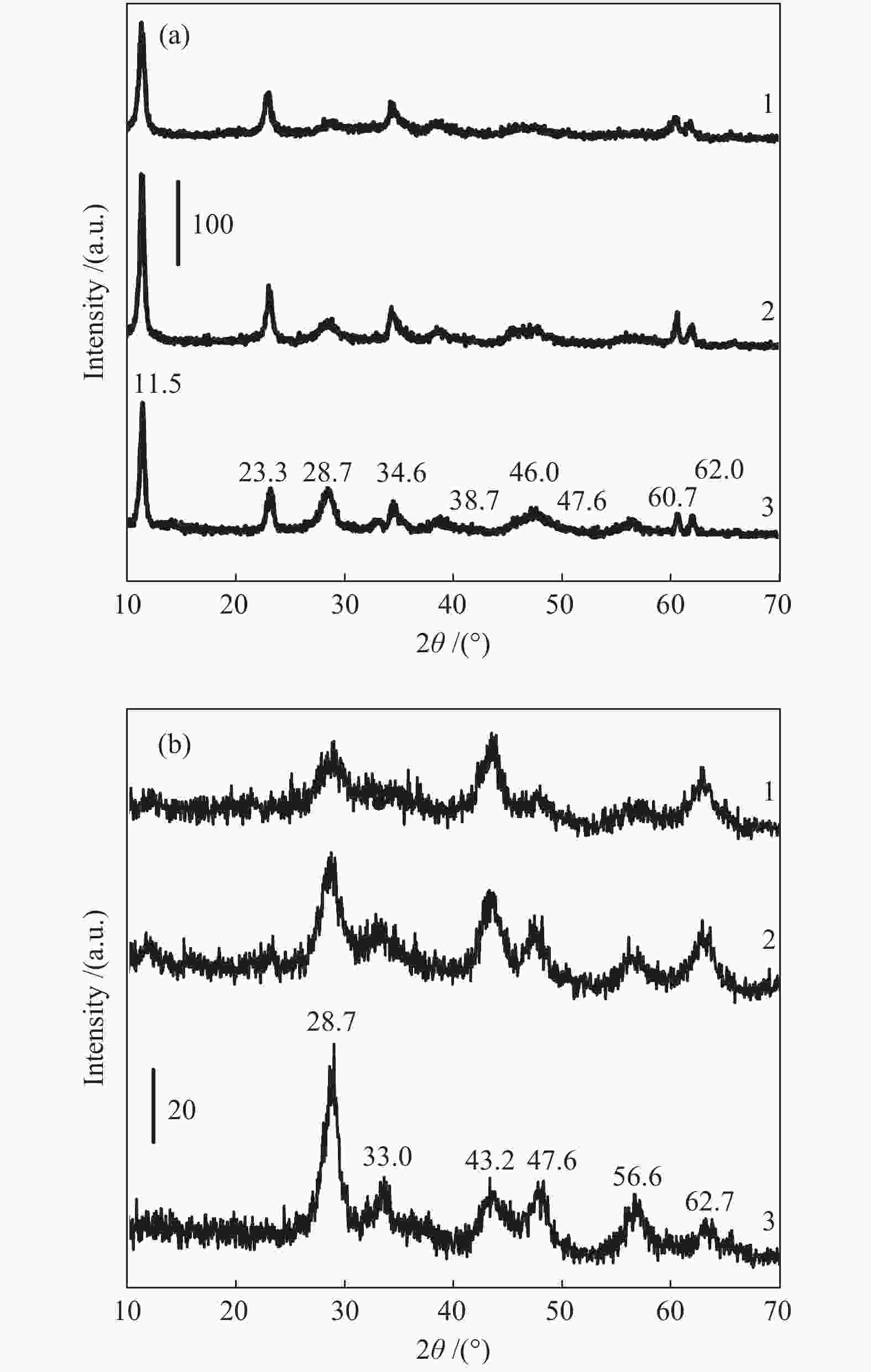
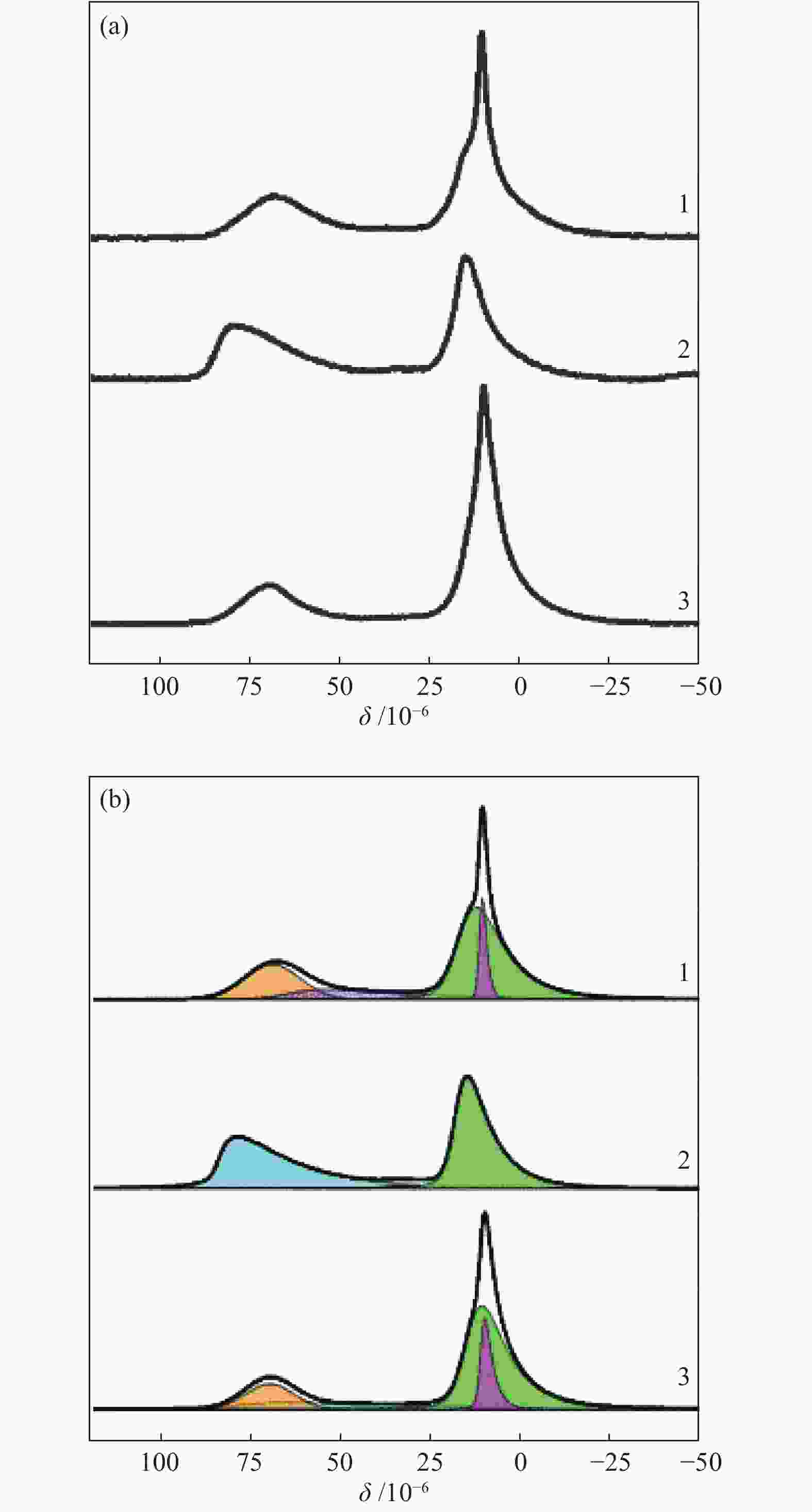
Table 1 27Al NMR analysis of Mg-Al-Ce oxide compositions
Sample Area/% Alocta 1, δ~11 Alocta 2,
δ = 14–18Alpenta,
δ = 33–35Altetra 1,
δ = 68–70Altetra 2,
δ = 80–84Mg-Al-Ce-4 9.4 53.7 − 15.1+21.8a − Mg-Al-Ce-2 − 50.6 1.4 − 48.0 Mg-Al-Ce-1 14.1 56.0 − 13.9 16.0 a: Altetra 2 signal for Mg-Al-Ce-4 is a sum of two components: δ1~65 and δ2 = 68–70 Table 2 Acid-base characteristics of the Mg-Al-Ce oxide catalysts
Characteristics Sitesa Catalyst samples [Ssp] Mg-Al-Ce-4
[78 m2·g−1]Mg-Al-Ce-2
[96 m2·g−1]Mg-Al-Ce-1
[122 m2·g−1]CeOx
[85 m2·g−1]Al2O3
[199 m2·g−1]MgO
[71 m2·g−1]Acid capacity/(${\rm{mmo}}{{\rm{l}}_{{\rm{NH}}}}_{_{\rm{3}}} \cdot {{\rm{g}}^{{\rm{ - 1}}}}$) sw 0.09 0.13 0.13 0.11 0.32 0.06 w 0.20 0.29 0.30 0.11 0.39 0.09 m 0.13 0.05 0.20 − s 0.14 0.16 0.08 0.23 − total 0.56 0.63 0.63 0.30 0.94 0.15 Acid density/(µ${\rm{mo}}{{\rm{l}}_{{\rm{NH}}}}_{_{\rm{3}}} \cdot {{\rm{m}}^{{\rm{ - 2}}}}$) sw 1.15 1.35 1.07 1.29 1.61 0.85 w 2.56 3.02 2.46 1.29 1.96 1.27 m 1.67 0.52 1.64 − s 1.79 1.67 0.94 1.16 − total 7.18 6.56 5.16 3.52 4.72 2.12 Base capacity/(${\rm{mmo} }{ {\rm{l} }_{ {\rm{CO} } } }_{_{\rm{2} } } \cdot {\rm{g} }$−1) sw 0.03 0.06 0.05 0.08 0.07 0.01 w 0.03 0.08 0.09 0.10 0.04 m 0.05 0.07 0.06 0.05 0.04 0.08 s 0.03 − 0.04 0.01 0.03 0.05 total 0.14 0.21 0.24 0.14 0.24 0.18 Base density/(µ${\rm{mo} }{ {\rm{l} }_{ {\rm{CO} } } }_{_{\rm{2} } } \cdot {\rm{m} }$−2) sw 0.38 0.63 0.41 0.94 0.35 0.14 w 0.38 0.83 0.74 0.50 0.56 m 0.64 0.73 0.49 0.59 0.20 1.13 s 0.38 − 0.33 0.12 0.15 0.70 total 1.79 2.19 1.97 1.65 1.21 2.53 Acid-base capacity ratio 4.0 3.0 2.6 2.1 3.9 0.8 a: abbreviations: “sw”: super weak sites, “w”: weak sites, “m”: medium sites, “s”: strong sites Table 3 Specific rate values for Mg-Al-Ce oxide catalysts in the process of vapour phase of ethanol conversion in a flow reactor (T = 548 K, TOS = 4 h, atmospheric pressure, WHSV = 0.14 g∙gcat−1∙h−1)
Sample Rate/(mol·m−2·s−1 × 1010) EtOH
conversionFormation BuOH HeOH AA Et DEE Mg-Al-Ce-4 11.381 0.775 0.015 4.557 0.224 0.149 Mg-Al-Ce-2 7.573 1.264 0.043 1.504 0.162 0.104 Mg-Al-Ce-1 6.514 0.458 0.015 3.487 0.214 0.117 -
[1] NDABA B, CHIYANZU I, MARX S. N-Butanol derived from biochemical and chemical routes: A review[J]. Biotechnol Reports,2015,8:1−9. doi: 10.1016/j.btre.2015.08.001 [2] RAJESH KUMAR B, SARAVANAN S. Use of higher alcohol biofuels in diesel engines: A review[J]. Renewable Sustainable Energy Rev,2016,60:84−115. doi: 10.1016/j.rser.2016.01.085 [3] UYTTEBROEK M, VAN HECKE W, VANBROEKHOVEN K. Sustainability metrics of 1-butanol[J]. Catal Today,2015,239:7−10. doi: 10.1016/j.cattod.2013.10.094 [4] ANGELICI C, WECKHUYSEN B M, BRUIJNINCX P C A. Chemocatalytic conversion of ethanol into butadiene and other bulk chemicals[J]. ChemSusChem,2013,6(9):1595−1614. doi: 10.1002/cssc.201300214 [5] GABRIËLS D, HERNÁNDEZ W Y, SELS B, VAN DER VOORT P, VERBERCKMOES A. Review of catalytic systems and thermodynamics for the Guerbet condensation reaction and challenges for biomass valorization[J]. Catal Sci Technol,2015,5:3876−3902. doi: 10.1039/C5CY00359H [6] O’LENICK Jr. AJ. Guerbet chemistry[J]. J Surfactants Deterg,2001,4(3):311−315. doi: 10.1007/s11743-001-0185-1 [7] NEZAM I, PEEREBOOM L, MILLER D J. Continuous condensed-phase ethanol conversion to higher alcohols: Experimental results and techno-economic analysis[J]. J Clean Prod,2019,209:1365−1375. doi: 10.1016/j.jclepro.2018.10.276 [8] DI COSIMO J I, DÍEZ V K, XU M, IGLESIA E, APESTEGUÍA C R. Structure and surface and catalytic properties of Mg-Al basic oxides[J]. J Catal,1998,178(2):499−510. doi: 10.1006/jcat.1998.2161 [9] ZHANG Q, DONG J, LIU Y, WANG Y, CAO Y. Towards a green bulk-scale biobutanol from bioethanol upgrading[J]. J Energy Chem,2016,25(6):8−11. [10] RAMASAMY K K, GRAY M, JOB H, SANTOSA D, LI X S, DEVARAJ A, KARKAMKAR A, WANG Y. Role of calcination temperature on the hydrotalcite derived MgO-Al2O3 in converting ethanol to butanol[J]. Top Catal,2016,59(1):46−54. doi: 10.1007/s11244-015-0504-8 [11] RAMASAMY K K, GRAY M, JOB H, SMITH C, WANG Y. Tunable catalytic properties of bi-functional mixed oxides in ethanol conversion to high value compounds[J]. Catal Today,2016,269:82−87. doi: 10.1016/j.cattod.2015.11.045 [12] CARVALHO D L, DE AVILLEZ R R, RODRIGUES M T, BORGES L E P, APPEL L G. Mg and Al mixed oxides and the synthesis of n-butanol from ethanol[J]. Appl Catal A: Gen,2012,415–416:96−100. [13] LEÓN M, DÍAZ E, ORDÓÑEZ S. Ethanol catalytic condensation over Mg-Al mixed oxides derived from hydrotalcites[J]. Catal Today,2011,164(1):436−442. doi: 10.1016/j.cattod.2010.10.003 [14] STOŠIĆ D, HOSOGLU F, BENNICI S, TRAVERT A, CAPRON M, DUMEIGNIL F, COUTURIER J, DUBOIS J, AUROUX A. Methanol and ethanol reactivity in the presence of hydrotalcites with Mg/Al ratios varying from 2 to 7[J]. Catal Commun,2017,89:14−18. doi: 10.1016/j.catcom.2016.10.013 [15] LARINA O V, VALIHURA K V, KYRIIENKO P I, VLASENKO N V, BALAKIN D Y, KHALAKHAN I, ČENDAK T, SOLOVIEV S O, ORLYK S M. Successive vapour phase Guerbet condensation of ethanol and 1-butanol over Mg-Al oxide catalysts in a flow reactor[J]. Appl Catal A: Gen,2019,588:117265. doi: 10.1016/j.apcata.2019.117265 [16] CHENG F L, GUO H Q, CUI J L, HOU B, LI D B. Guerbet reaction of methanol and ethanol catalyzed by CuMgAlOx mixed oxides: Effect of M2+/Al3+ ratio[J]. J Fuel Chem Technol,2018,46(12):1472−1481. doi: 10.1016/S1872-5813(18)30061-6 [17] MARCU I C, TANCHOUX N, FAJULA F, TICHIT D. Catalytic conversion of ethanol into butanol over M-Mg-Al mixed oxide catalysts (M = Pd, Ag, Mn, Fe, Cu, Sm, Yb) obtained from LDH precursors[J]. Catal Lett,2013,143(1):23−30. doi: 10.1007/s10562-012-0935-9 [18] MARCU I C, TICHIT D, FAJULA F, TANCHOUX N. Catalytic valorization of bioethanol over Cu-Mg-Al mixed oxide catalysts[J]. Catal Today,2009,147(3/4):231−238. [19] CARLINI C, MARCHIONNA M, NOVIELLO M, RASPOLLI GALLETTI A M, SBRANA G, BASILE F, VACCARI A. Guerbet condensation of methanol with n-propanol to isobutyl alcohol over heterogeneous bifunctional catalysts based on Mg-Al mixed oxides partially substituted by different metal components[J]. J Mol Catal A: Chem,2005,232(1/2):13−20. [20] HERNANDEZ W Y, De VLIEGER K, VAN DER VOORT P, VERBERCKMOES A. Ni-Cu Hydrotalcite-derived mixed oxides as highly selective and stable catalysts for the synthesis of β-branched bioalcohols by the Guerbet Reaction[J]. ChemSusChem,2016,9(22):3196−3205. doi: 10.1002/cssc.201601042 [21] BENITO P, VACCARI A, ANTONETTI C, LICURSI D, SCHIARIOLI N, RODRIGUEZ-CASTELLÓN E, RASPOLLI GALLETTI A M. Tunable copper-hydrotalcite derived mixed oxides for sustainable ethanol condensation to n-butanol in liquid phase[J]. J Clean Prod,2019,209:1614−1623. doi: 10.1016/j.jclepro.2018.11.150 [22] WANG Z, FONGARLAND P, LU G, ESSAYEM N. Reconstructed La-, Y-, Ce-modified MgAl-hydrotalcite as a solid base catalyst for aldol condensation: Investigation of water tolerance[J]. J Catal,2014,318:108−118. doi: 10.1016/j.jcat.2014.07.006 [23] DIEZ A S, GRAZIANO-MAYER M, RADIVOY G, VOLPE M A. Suzuki-Miyaura cross-coupling of aryl iodides and phenylboronic acid over palladium-free CeO2 catalysts[J]. Appl Catal A: Gen,2014,482:24−30. doi: 10.1016/j.apcata.2014.05.020 [24] WU X, FANG G, LIANG Z, LENG W, XU K, JIANG D, NI J, LI X. Catalytic upgrading of ethanol to n-butanol over M-CeO2/AC (M = Cu, Fe, Co, Ni and Pd) catalysts[J]. Catal Commun,2017,100:15−18. doi: 10.1016/j.catcom.2017.06.016 [25] VLASENKO N V, KYRIIENKO P I, VALIHURA K V, YANUSHEVSKA O I, SOLOVIEV S O, STRIZHAK P E. Effect of modifying additives on the catalytic properties of zirconium dioxide in the conversion of ethanol into 1-butanol[J]. Theor Exp Chem,2019,55(1):43−49. doi: 10.1007/s11237-019-09594-6 [26] VLASENKO N V, KYRIIENKO P I, YANUSHEVSKA O I, VALIHURA K V, SOLOVIEV S O, STRIZHAK P E. The effect of ceria content on the acid-base and catalytic characteristics of ZrO2-CeO2 oxide compositions in the process of ethanol to n-butanol condensation[J]. Catal Lett,2020,150(1):234−242. doi: 10.1007/s10562-019-02937-x [27] D’ESPINOSEDE LACAILLERIE J B, FRETIGNY C, MASSIOT D. MAS NMR spectra of quadrupolar nuclei in disordered solids: The Czjzek model[J]. J Magn Reson,2008,192(2):244−251. doi: 10.1016/j.jmr.2008.03.001 [28] VLASENKO N V, KOCHKIN Y N, PUZIY A M. Liquid phase synthesis of ethyl-tert-butyl ether: The relationship between acid, adsorption and catalytic properties of zeolite catalysts[J]. J Mol Catal A: Chem,2006,253(1/2):192−197. [29] KAPUSTIN G I, BRUEVA T R. A simple method for determination of heats of ammonia adsorption on catalysts from thermodesorption data[J]. Thermochim Acta,2001,379(1/2):71−75. [30] CANTRELL D G, GILLIE L J, LEE A F, WILSON K. Structure-reactivity correlations in MgAl hydrotalcite catalysts for biodiesel synthesis[J]. Appl Catal A: Gen,2005,287:183−190. doi: 10.1016/j.apcata.2005.03.027 [31] HE L, LI J, FENG Z, SUN D, WANG T, LI R, XU Y. Solvothermal synthesis and characterization of ceria with solid and hollow spherical and multilayered morphologies[J]. Appl Surf Sci,2014,322:147−154. doi: 10.1016/j.apsusc.2014.10.100 [32] ŚWIRK K, RØNNING M, MOTAK M, BEAUNIER P, DA COSTA P, GRZYBEK T. Ce- and Y-modified double-layered hydroxides as catalysts for dry reforming of methane: on the effect of yttrium promotion[J]. Catalysts,2019,9(1):56. doi: 10.3390/catal9010056 [33] PARAMESWARAM G, RAO P S N, SRIVANI A, RAO G N, LINGAIAH N. Magnesia-ceria mixed oxide catalysts for the selective transesterification of glycerol to glycerol carbonate[J]. Mol Catal,2018,451:135−142. doi: 10.1016/j.mcat.2017.12.006 [34] XU Z, LU G. Hydrothermal synthesis of layered double hydroxides (LDHs) from mixed MgO and Al2O3: LDH formation mechanism[J]. Chem Mater,2005,17(5):1055−1062. doi: 10.1021/cm048085g [35] KIKHTYANIN O, ČAPEK L, SMOLÁKOVÁ L, TIŠLER Z, KADLEC D, LHOTKA M, DIBLÍKOVÁ P, KUBIČKA D. Influence of Mg-Al mixed oxide compositions on their properties and performance in aldol condensation[J]. Ind Eng Chem Res,2017,56(45):13411−13422. doi: 10.1021/acs.iecr.7b03367 [36] COENEN K, GALLUCCI F, MEZARI B, VERHOEVEN T, HENSEN E, VAN SINT ANNALAND M. Investigating the role of the different metals in hydrotalcite Mg/Al-based adsorbents and their interaction with acidic sorbate species[J]. Chem Eng Sci,2019,200:138−146. doi: 10.1016/j.ces.2019.01.046 [37] PAPARAZZO E. Use and mis-use of X-ray photoemission spectroscopy Ce3d spectra of Ce2O3 and CeO2[J]. J Phys Condens Matter,2018,30:343003. doi: 10.1088/1361-648X/aad248 [38] STETSOVYCH V, PAGLIUCA F, DVOŘÁK F, DUCHON T, VOROKHTA M, AULICKÁ M, LACHNITT J, SCHERNICH S, MATOLÍNOVÁ I, VELTRUSKÁ K, SKÁLA T, MAZUR D, MYSLIVEČEK J, LIBUDA J, MATOLÍN V. Epitaxial cubic Ce2O3 films via Ce-CeO2 interfacial reaction[J]. J Phys Chem Lett,2013,4:866−871. doi: 10.1021/jz400187j [39] DAMYANOVA S, BUENO J M C. Effect of CeO2 loading on the surface and catalytic behaviors of CeO2-Al2O3-supported Pt catalysts[J]. Appl Catal A: Gen,2003,253:135−150. doi: 10.1016/S0926-860X(03)00500-3 [40] ROMEO M, BAK K, FALLAH J EL, NORMAND F LE, HILAIRE L, LEVELS CC. XPS study of the reduction of cerium dioxide[J]. Surf Interface Anal,1993,20:508−512. doi: 10.1002/sia.740200604 [41] SKÁLA T, MATOLÍN V. Model thin films of Ce(III)-based mixed oxides[J]. Surf Interface Anal,2014,46(10/11):993−996. [42] WANG L J, LIU Y Q, WANG Q, CHOU K C. Evolution mechanisms of MgO·Al2O3 inclusions by cerium in spring steel used in fasteners of high-speed railway[J]. ISIJ Int,2015,55(5):970−975. doi: 10.2355/isijinternational.55.970 [43] AL-DOGHACHIA F A J, RASHIDC U, ZAINALA Z, SAIMANA M I, YAP Y H T. Influence of the Ce2O3 and CeO2 promoters on the Pd/MgO catalyst in dry-reforming of methane[J]. RSC Adv,2015,5:81739−81752. doi: 10.1039/C5RA15825G [44] ZOU W, GE C, LU M, WU S, WANG Y, SUN J, P U Y, TANG C, GAO F, DONG L. Engineering the NiO/CeO2 interface to enhance the catalytic performance for CO oxidation[J]. RSC Adv,2015,5(119):98335−98343. doi: 10.1039/C5RA20466F [45] GUO M, LU J, WU Y, WANG Y, LUO M. UV and visible Raman studies of oxygen vacancies in rare-earth-doped ceria[J]. Langmuir,2011,27(7):3872−3877. doi: 10.1021/la200292f [46] VINOD KUMAR T, MUKHERJEE D, SUBRAHMANYAM C, REDDY B M. Investigation on the physicochemical properties of Ce0.8Eu0.1M0.1O2-δ (M = Zr, Hf, La, and Sm) solid solutions towards soot combustion[J]. New J Chem,2018,42(7):5276−5783. doi: 10.1039/C8NJ00007G [47] REDDY B M, BHARALI P, SAIKIA P, PARK S E, VAN DEN BERG M W E, MUHLER M, GRÜNERT W. Structural characterization and catalytic activity of nanosized CexM1-xO2 (M = Zr and Hf) mixed oxides[J]. J Phys Chem C,2008,112(31):11729−11737. doi: 10.1021/jp802674m [48] BENSALEM A, BOZON-VERDURAZ F, DELAMAR M, BUGLI G. Preparation and characterization of highly dispersed silica-supported ceria[J]. Appl Catal A: Gen,1995,121(1):81−93. doi: 10.1016/0926-860X(94)00193-6 [49] ZAKI M I, HUSSEIN G A M, MANSOUR S A A, ISMAIL H M, MEKHEMER G A H. Ceria on silica and alumina catalysts: Dispersion and surface acid- base properties as probed by X-ray diffractometry, UV-Vis diffuse reflectance and in situ IR absorption studies[J]. Colloids Surfaces A Physicochem Eng Asp,1997,127(1/3):47−56. -
 2020-W023-supporting information.pdf
2020-W023-supporting information.pdf
-





 下载:
下载: