Catalytic performance of different zeolites for propane and CO2 coupling to propylene
-
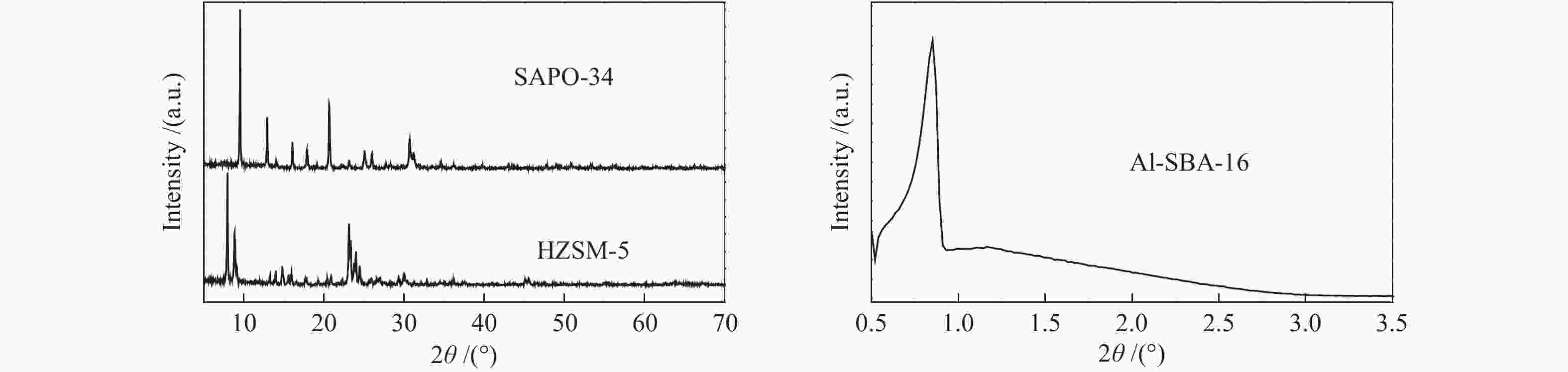
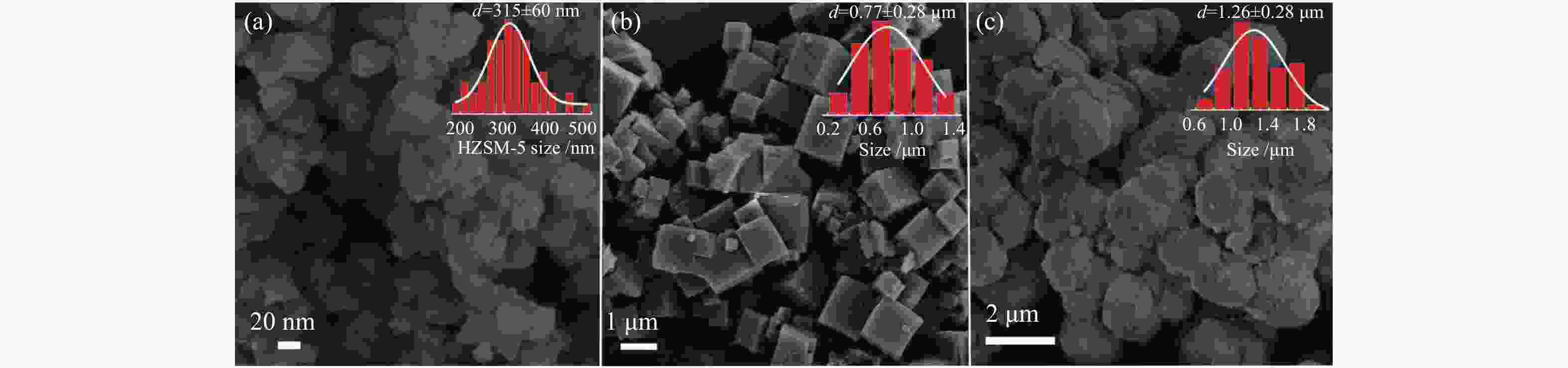
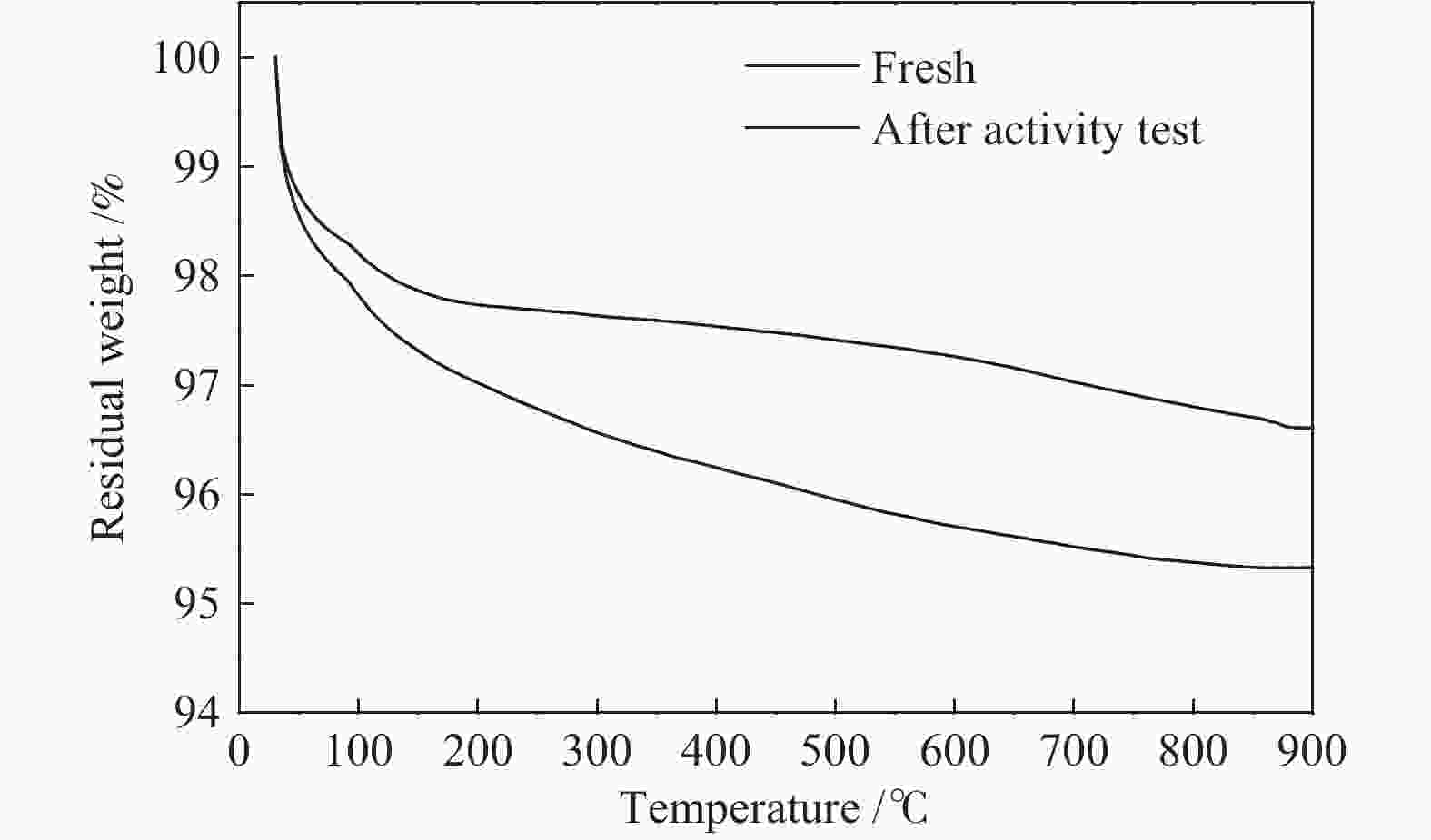
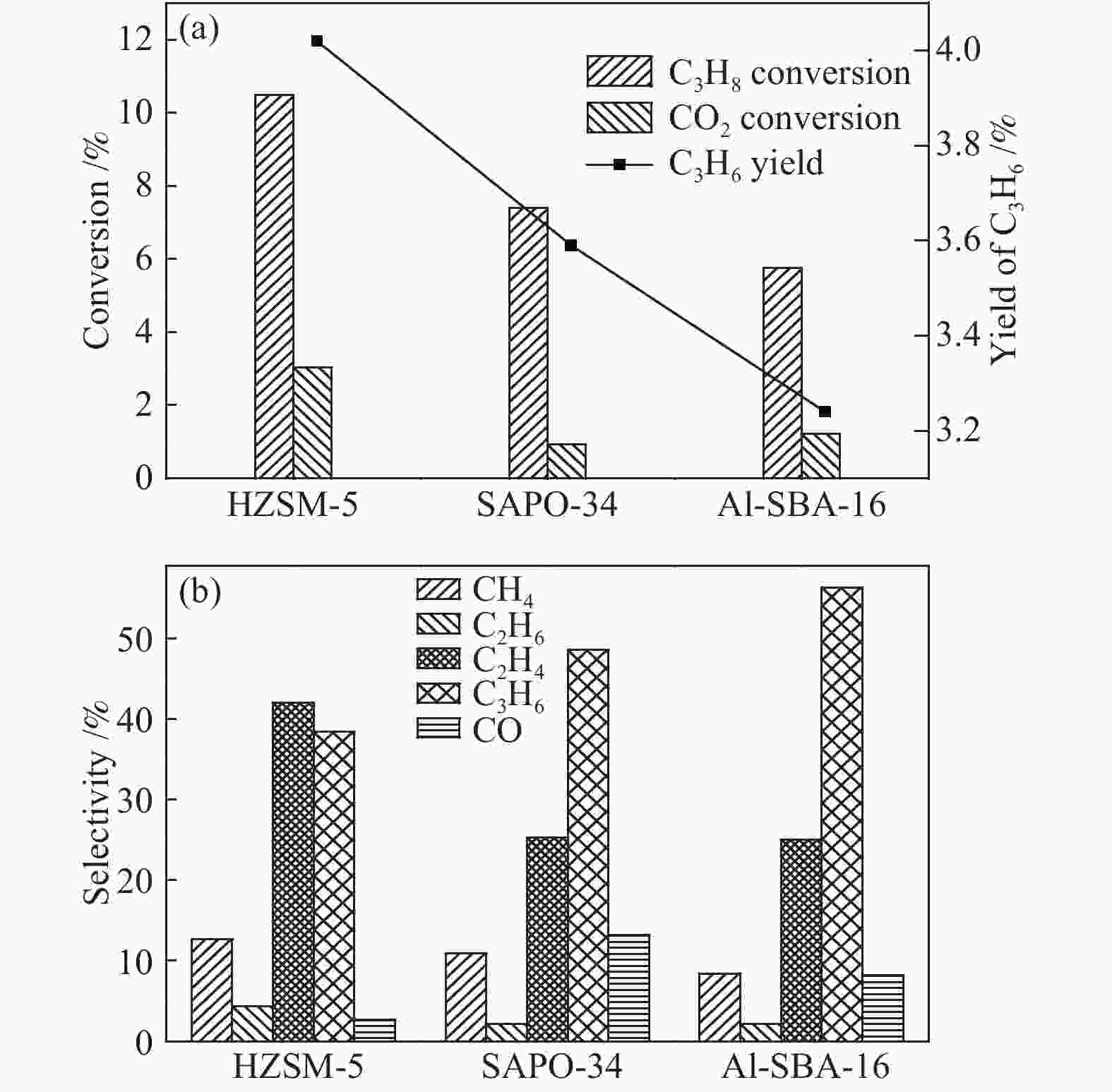
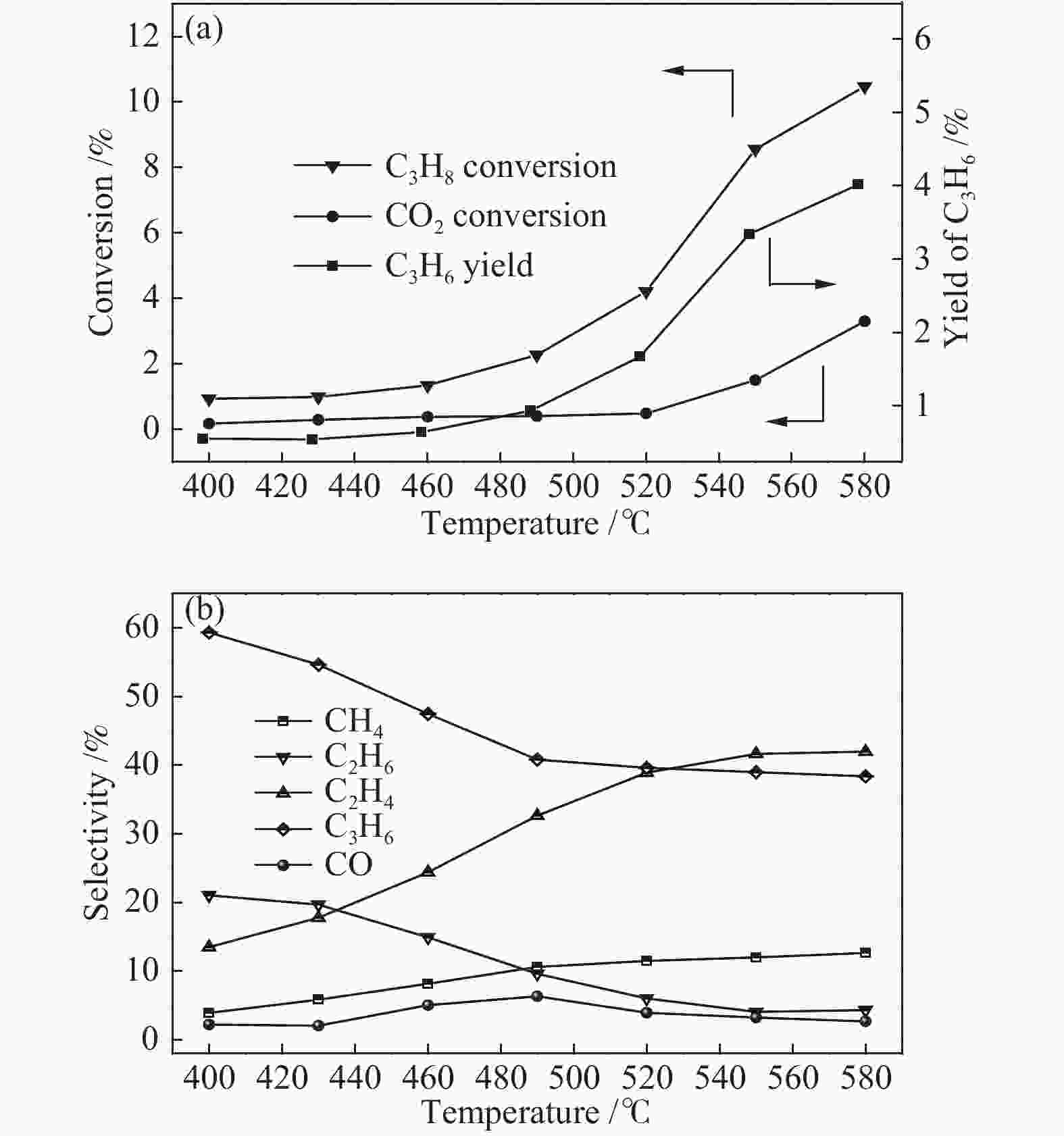
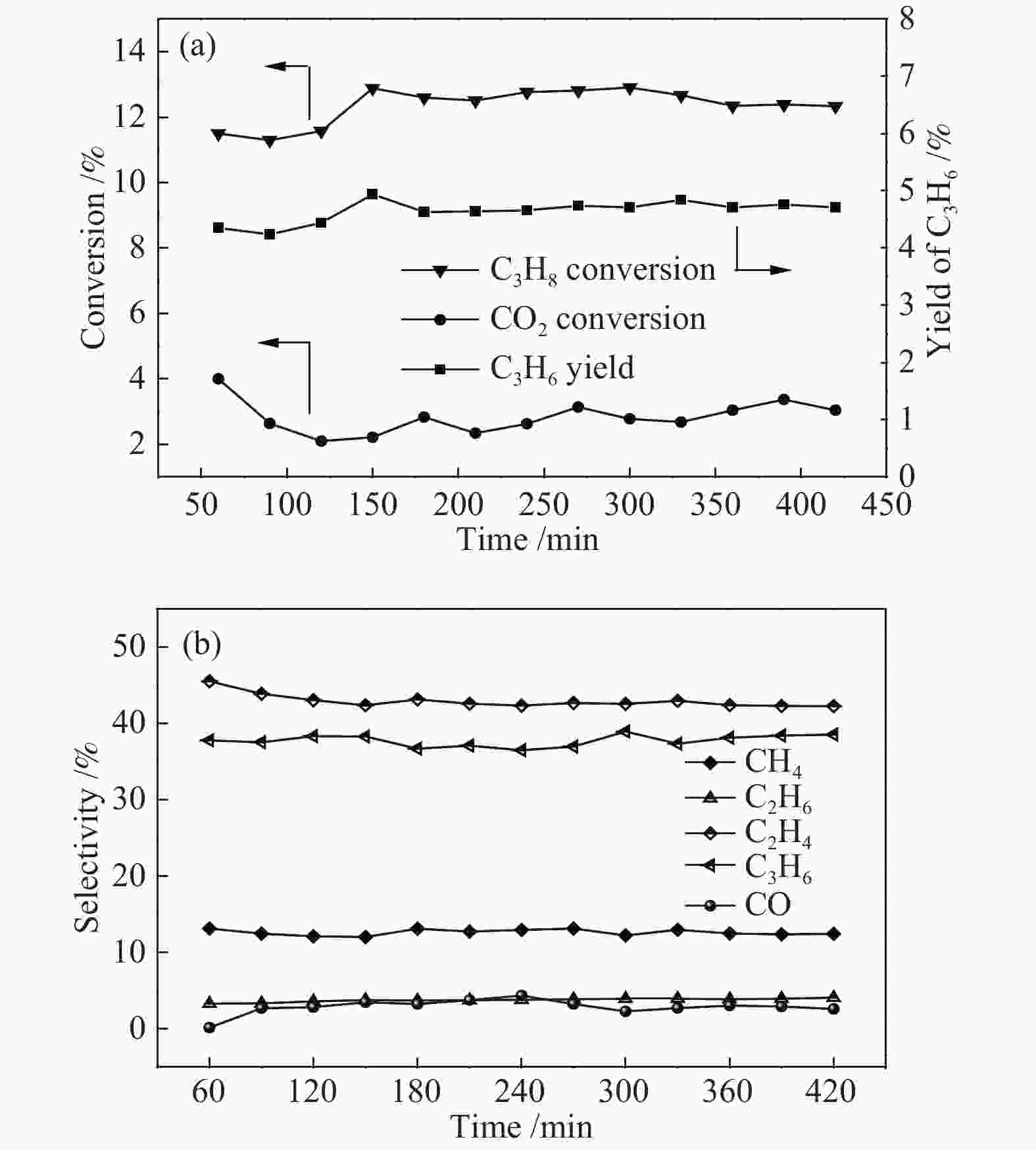
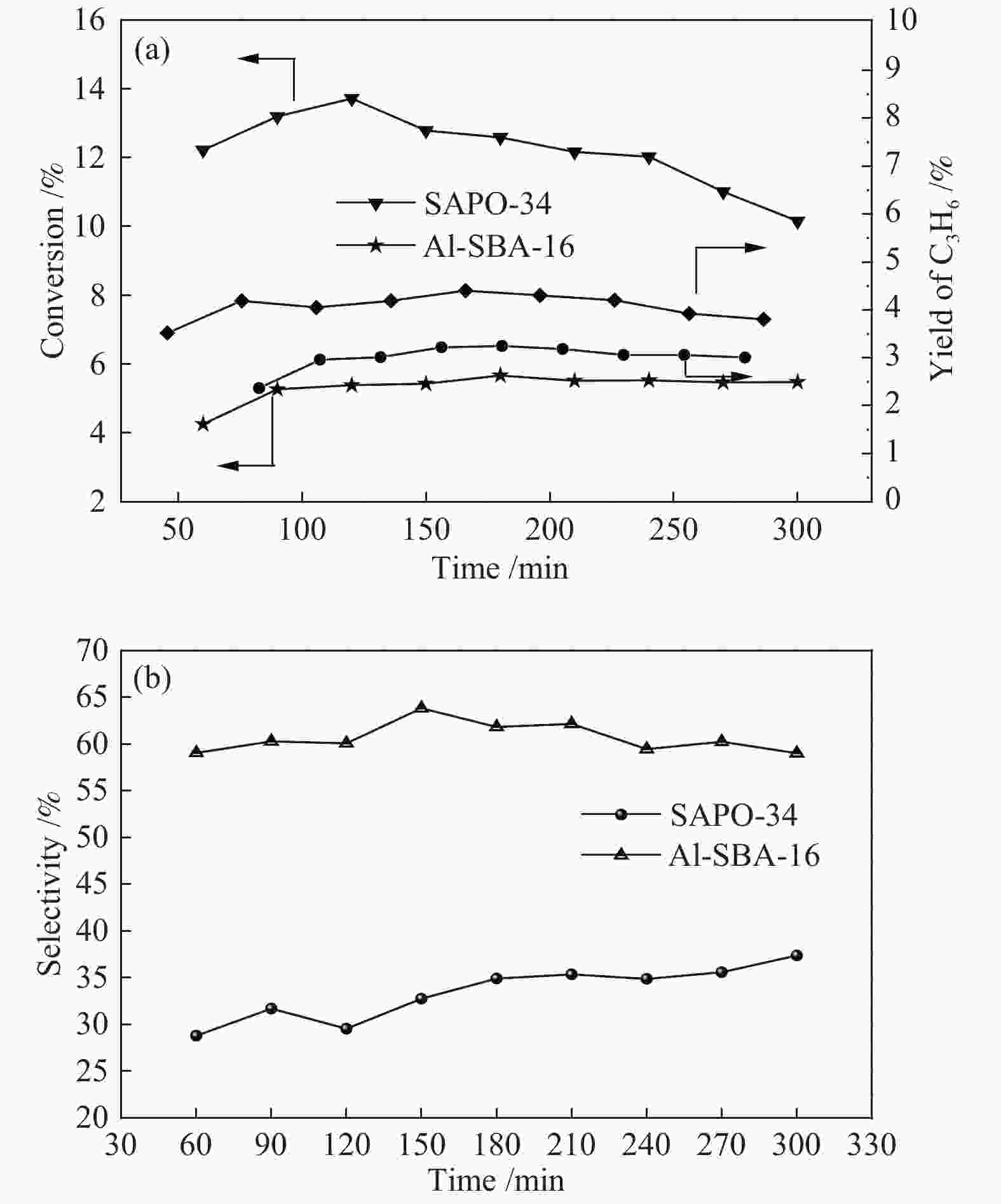
摘要: 丙烷脱氢制丙烯为吸热反应,同时伴随氢气的生成,而CO2加氢制低碳烯烃为放热反应,因此,将这两个反应耦合,可打破单一反应在热力学和动力学上的平衡限制,提高丙烯的产率。在此基础上进一步探究了不同晶型分子筛(HZSM-5、SAPO-34和Al-SBA-16)对丙烷与CO2耦合制丙烯反应性能的影响。通过XRD、SEM、NH3-TPD、N2吸附-脱附、TG等手段对不同晶型分子筛的性质进行表征,并在固定床反应器上考察了三种分子筛在丙烷和二氧化碳耦合制丙烯反应中的催化性能。实验结果表明,HZSM-5分子筛弱酸位点含量较高、比表面积大,且展现出优异的催化性能。当丙烷与CO2的体积比为1: 4,反应压力为0.1 MPa,反应温度为580 ℃,催化剂用量为0.2 g,空速为6000 mL/(gcat·h)时,丙烷转化率为10.5%,CO2转化率为3.0%,丙烯选择性为38.4%、产率为4.0%。Abstract: CO2 hydrogenation to light olefins is an exothermic reaction, while propane dehydrogenation to propylene is an endothermic reaction, and with generating of hydrogen. Coupling of the two reactions can break the equilibrium limit of thermodynamics and dynamics of the single reaction and improve yield of propylene. Therefore, the effects of different crystalline zeolites (HZSM-5, SAPO-34 and Al-SBA-16) on the reactivity of propane coupled with CO2 to propylene were investigated. The properties of different zeolites were characterized by means of XRD, SEM, NH3-TPD, N2 desorption and TG, and the catalytic performance of the three different zeolites in the reaction of propane and carbon dioxide coupling to propylene was investigated in a fixed bed reactor. The experimental results showed that HZSM-5 zeolite had high content of weak acid, large specific surface area and excellent catalytic activity. Typically the propane conversion rate is 10.5%, the CO2 conversion is 3.0%, the propylene selectivity is 38.4 and the yield is 4.0% when the volume ratio of propane to CO2 is 1:4, the reaction pressure is 0.1 MPa, the reaction temperature is 580 ℃, the catalyst mass is 0.2 g and the space velocity is 6000 mL/(gcat·h).
-
Key words:
- zeolites /
- coupling reaction /
- propane /
- carbon dioxide /
- propylene
-
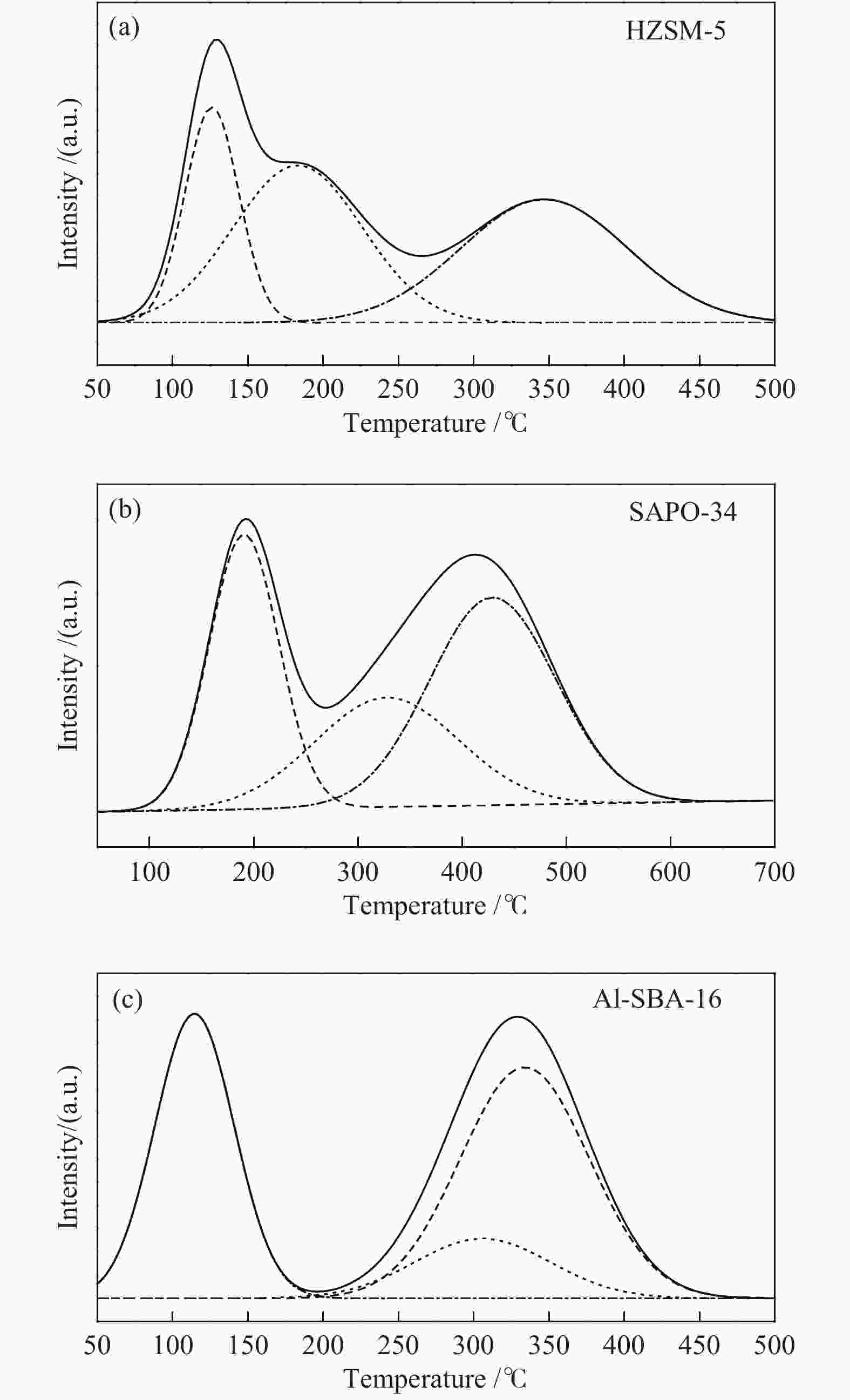
表 1 催化剂的酸浓度和酸位强度
Table 1 Concentration and strength of acid sites of catalysts
Sample Concentration of
acid sites (a.u.·g−1)Strength of
acid sites/℃weak medium strong weak medium strong HZSM-5 75 132 134 126 183 347 SAPO-34 1058 866 1445 191 327 429 Al-SBA-16 26 10 35 114 306 340 表 2 催化剂的结构特性
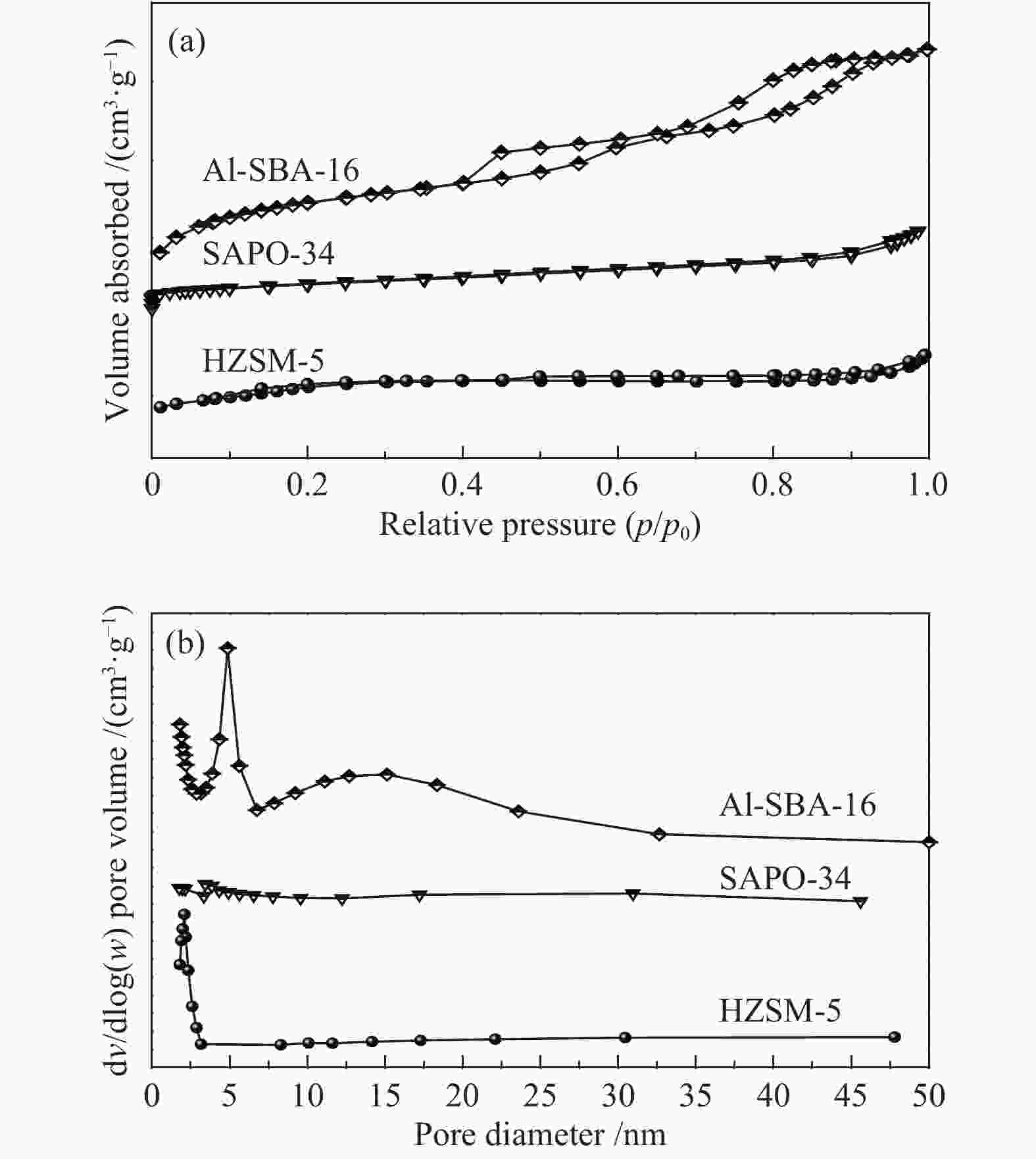
Table 2 Textural properties of the catalysts
Sample SBET/
(m2·g−1)Smic/
(m2·g−1)Smes/
(m2·g−1)vmic/
(cm3·g−1)vtotal/
(cm3·g−1)HZSM-5 326.50 193.43 133.07 0.104 0.194 SAPO-34 219.67 200.97 18.71 0.117 0.119 Al-SBA-16 274.25 80.55 193.70 0.043 0.279 SBET (surface area calculated by BET), Smic (surface area of micropores by t-plot method),Smes = SBET − Smic, vmic (micropore volume by t-plot method), vtotal ((total pore volume) -
[1] 吴锁林. 丙烷脱氢制丙烯的技术进展[J]. 江苏化工,1998,26(2):35−37.WU Suo-lin. Technologic advances preparing propylene by propane dehydrogenation[J]. Jiangsu Chem,1998,26(2):35−37. [2] WANG J Y, ZHANG A F, JIANG X, SONG C S, GUO X W. Highly selective conversion of CO2 to lower hydrocarbons (C2-C4) over bifunctional catalysts composed of In2O3-ZrO2 and zeolite[J]. J CO2 Util,2018,27:81−88. doi: 10.1016/j.jcou.2018.07.006 [3] ZHU H B, ANJUM D H, WANG Q X, ABOU-HAMAD E, EMSLEY L, DONG H L, LAVEILLE P, LI L D, SAMAL A K, BASSET J M. Sn surface-enriched Pt-Sn bimetallic nanoparticles as a selective and stable catalyst for propane dehydrogenation[J]. J Catal,2014,320:52−62. doi: 10.1016/j.jcat.2014.09.013 [4] ZHU J, YANG M L, YU Y D, Zhu Y A, SUI Z J, ZHOU X G, HOLMEN A, CHEN D. Size-dependent reaction mechanism and kinetics for propane dehydrogenation over Pt catalysts[J]. ACS Catal,2015,5(11):6310−6319. doi: 10.1021/acscatal.5b01423 [5] AIRAKSINEN S M, KRAUSE A O I. effect of catalyst prereduction on the dehydrogenation of isobutane over chromia/alumina[J]. Ind Eng Chem Res,2005,44(11):3862−3868. doi: 10.1021/ie050060j [6] 葛欣. 丙烷脱氢耦合逆水煤气变换制丙烯催化反应研究进展[J]. 天然气化工 (C1化学与化工),2010,35(5):61−66.GE Xin. Progress in catalytic reaction of propane dehydrogenation coupled reverse water-gas conversion to propylene[J]. Natural Gas Chem,2010,35(5):61−66. [7] SHEN K, QIAN W Z, WANG N, SU C, WEI F. Fabrication of c-axis oriented ZSM-5 hollow fifibers based on an in situ solid-solid transformation mechanism[J]. J Am Chem Soc,2013,135(47):15322−15325. [8] BAI L Y, ZHOU Y M, ZHANG Y W, LIU H, SHENG X L. Effect of magnesium addition to PtSnNa/ZSM-5 on the catalytic properties in the dehydrogenation of propane[J]. Ind Eng Chem Res,2009,48(22):9885−9891. doi: 10.1021/ie900534m [9] SU X F, ZAN W, BAI X F. Synthesis of microscale and nanoscale ZSM-5 zeolites: Effect of particle size and acidity of Zn modified ZSM-5 zeolites on aromatization performance[J]. Cata Sci Technol,2017,7(9):1943−1952. doi: 10.1039/C7CY00435D [10] ZHANG F, MIAO C X, YUE Y H. Dehydrogenation of propane to propylene in the presence of CO2 over steaming-treated HZSM-5 supported ZnO[J]. Chin J Chem,2012,30(4):929−934. doi: 10.1002/cjoc.201100379 [11] NAWAZ Z, TANG X P, ZHANG Q, WANG D Z, WEI F. SAPO-34 supported Pt-Sn-based novel catalyst for propane dehydrogenation to propylene[J]. Catal Commun,2009,10(14):1925−1930. doi: 10.1016/j.catcom.2009.07.008 [12] CHEN J S, WRIGHT P A, THOMAS J M, NATARAJAN S, MARCHESE L, BRADLEY S M, SANKAR G, CATLOW C R A. SAPO-18 catalysts and their brönsted acid sites[J]. J Phys Chem,1994,98(40):10216−10224. doi: 10.1021/j100091a042 [13] SASTRE G, LEWIS D W, CATIOW C R A. Modeling of silicon substitution in SAPO-5 and SAPO-34 molecular sieves[J]. J Phys Chem,1997,101(27):5249−5262. doi: 10.1021/jp963736k [14] NAWAZ Z, TANG X P, WANG Y, WEI F. Parametric characterization and influence of tin on theperformance of Pt-Sn/SAPO-34 catalyst for selective propane dehydrogenation to propylene[J]. Ind Eng Chem Res,2010,49(3):1274−1280. doi: 10.1021/ie901465s [15] PERAL A, ESCOLA J M, SERRANO D P, PRECH J, OCHOA-HERNANDEZ C. Bidimensional ZSM-5 zeolites probed as catalysts for polyethylene cracking[J]. Catal Sci Technol,2016,6(8):2754−2765. doi: 10.1039/C5CY02075A [16] YAN Y E, GUO X, ZHANG Y H, TANG Y. Future of nano-hierarchical zeolites in catalysis: Gaseous phase or liquid phase system[J]. Catal Sci Technol,2015,5(2):772−785. doi: 10.1039/C4CY01114G [17] LIU Y M, XIE S H, CAO Y, HE H Y. Synthesis of novel cage-like mesoporous vanadosilicate and its efficient performance for oxidation dehydrogenation of propane[J]. J Phys Chem,2010,114(13):5941−5946. [18] ZHANG L, DENG J G, DAI H X, AU C T. Binary Cr-Mo oxide catalysts supported on MgO-coated polyhedral three-dimensional mesoporous SBA-16 for the oxidative dehydrogenation of iso-butane[J]. Appl Catal A: Gen,2009,354(1):72−81. [19] CHEN X X, XI D Y, SUN Q M, WANG N, DAI Z Y, FAN D, VALTCHEV V, YU J H. A top-down approach to hierarchical SAPO-34 zeolites with improved selectivity of olefin[J]. Microporous Mesoporous Mater,2016,234:401−408. doi: 10.1016/j.micromeso.2016.07.045 [20] GAO Y, WU G, MA F W, LIU C T, JIANG F, WANG Y, WANG A J. Modified seeding method for preparing hierarchical nanocrystalline ZSM-5 catalysts for methanol aromatization[J]. Microporous Mesoporous Mater,2016,226:251−259. doi: 10.1016/j.micromeso.2015.11.066 [21] HUIRACHE-ACUNA R, PAWELEC B, LORICERA C V, RIVERA-MUNOZ, NAVA R, TORRES B, FIERRO J L G. Comparison of the morphology and HDS activity of ternary Ni (Co)-Mo-W catalysts supported on Al-HMS and Al-SBA-16 substrates[J]. Appl Catal B: Environ,2012,125:473−485. doi: 10.1016/j.apcatb.2012.05.034 [22] ZHAANG S H, MURATSUGU S, ISHIGURO N, TADA M. Ceria-doped Ni/SBA-16 catalysts for dry reforming of methane[J]. ACS Catal,2013,3(8):1855−1864. doi: 10.1021/cs400159w [23] 龙丽, 肖松, 李志毅. SAPO-34分子筛的制备与表征[J]. 硅酸盐通报,2015,34(4):914−919.LONG Li, XIAO Song, LI Zhi-yi. Preparation and characterization of nano SAPO-34 molecular sieve[J]. Bull Chin Ceramic Soc,2015,34(4):914−919. [24] 康琪琪. SAPO-34合成、改性及其丁烯裂解催化性能的研究[D]. 大庆: 东北石油大学, 2018.KANG Qi-qi. study on synthesis and modification of SAPO-34 and catalytic performance of butene cracking[D]. Daqing: Northeast Petroleum University, 2018. [25] SUN L Y, WANG Y Q, CHEN H B, SUN C, MENG F J, GAO F, WANG X. Direct synthesis of hierarchical ZnZSM-5 with addition of CTAB in a seeding method and improved catalytic performance in methanol to aromatics reaction[J]. Catal Today,2018,316:91−98. doi: 10.1016/j.cattod.2018.01.015 [26] NIU X J, GAO J, MIAO Q, DONG M, WANG G F, FAN W B, QIN Z F, WANG J G. Influence of preparation method on the performance of Zn-containing HZSM-5 catalysts in methanol-to-aromatics[J]. Microporous Mesoporous Mater,2014,197:252−261. doi: 10.1016/j.micromeso.2014.06.027 [27] 张同旺, 武雪峰, 侯拴弟. 催化剂积炭对甲醇制低碳烯烃效果的影响[J]. 石油加工,2011,27(6):891−896.ZHANG Tong-wang, WU Xue-feng, HOU Shuan-di. The effect of catalyst carbon deposition on the preparation of low carbon olefin from methanol[J]. Pet Process Sect,2011,27(6):891−896. [28] DANG S, LI S G, YANG C G, CHEN X Q, LI X P, ZHONG L S, GAO P, SUN Y H. sElective transformation of CO2 and H2 into lower olefins over In2O3-ZnZrOx/SAPO-34 bifunctional catalysts[J]. ChemSusChem,2019,12(15):3582−3591. doi: 10.1002/cssc.201900958 [29] NI Y M, SUN A M, WU X L, Hai G L, HU J L, LI T, LI G X. The preparation of nano-sized H[Zn, Al]ZSM-5 zeolite and its application in the aromatization of methanol[J]. Microporous Mesoporous Mater,2011,143(2-3):435−442. doi: 10.1016/j.micromeso.2011.03.029 [30] VALTCHEV V, BALANZAT E, MAVRODINOVA V, DIAZ L, El FALLAH J, GOUPIL J M. High energy ion irradiation-induced ordered macropores in zeolite crystals[J]. J Am Chem Soc,2011,133(46):18950−18956. doi: 10.1021/ja208140f [31] LU P, SUN J, SHEN D, YANG R Q, XING C, LU C X, TSUBAKI N, SHAN S D. Direct syngas conversion to liquefied petroleum gas: importance of a multifunctional metal-zeolite interface[J]. Appl Energy,2018,209(1):1−7. [32] HAN Z F, XUE X L, WU J M, LANG W Z, GUO Y J. Preparation and catalytic properties of mesoporous nV-MCM-41 for propane oxidative dehydrogenation in the presence of CO2[J]. Chin J Catal,2018,39(6):1099−1109. doi: 10.1016/S1872-2067(18)63048-7 [33] REN G Q, PEI G X, REN Y J, LIU K P, CHEN Z Q, YANG J Y, SU Y, LIU X Y, LI W Z. Effect of group IB metals on the dehydrogenation of propane to propylene over anti-sintering Pt/MgAl2O4[J]. J Catal,2018,366:115−126. doi: 10.1016/j.jcat.2018.08.001 [34] 耿潭. 丙烷无氧催化脱氢PtSn/SBA-16催化剂的研究[D]. 北京: 中国石油大学 (北京), 2018.GEN Tan. Study on PtSn/SBA-16 catalyst for anaerobic catalytic dehydrogenation of propane[D]. Beijing: China University of Petroleum (Beijing), 2018. [35] BENCO L, BUCKO T, HAFER J. Dehydrogenation of propane over ZnMOR. static and dynamic reaction energy diagram[J]. J Catal,2011,277(1):104−116. doi: 10.1016/j.jcat.2010.10.018 [36] ZHANG Y W, ZHOU Y M, HUANG L, ZHOU S J, SHENG X L, WANG Q L, ZHANG C. Structure and catalytic properties of the Zn-modified ZSM-5 supported platinum catalyst for propane dehydrogenation[J]. Chem Eng J,2015,270:352−361. doi: 10.1016/j.cej.2015.01.008 [37] GUISNET M, GNEP N S. Aromatization of propane over GaHMFI catalysts reaction scheme, nature of the dehydrogenating species and mode of coke formation[J]. Catal Today,1996,31(3/4):275−292. doi: 10.1016/S0920-5861(96)00018-1 [38] LIU H, ZHOU Y, ZHANG Y, BAI L Y, TANG M H. Influence of binder on the catalytic performance of PtSnNa/ZSM-5 catalyst for propane dehydrogenation[J]. Ind Eng Chem Res,2008,47(21):8142−8147. doi: 10.1021/ie800693t [39] OGONOWSKI J, SKRZYNSKA E. Conversion of lower hydrocarbons in the presence of carbon dioxide: the theoretic analysis and catalytic tests over active carbon supported vanadium oxide[J]. Catal Lett,2008,124(1/2):52−58. doi: 10.1007/s10562-008-9438-0 -




 下载:
下载: