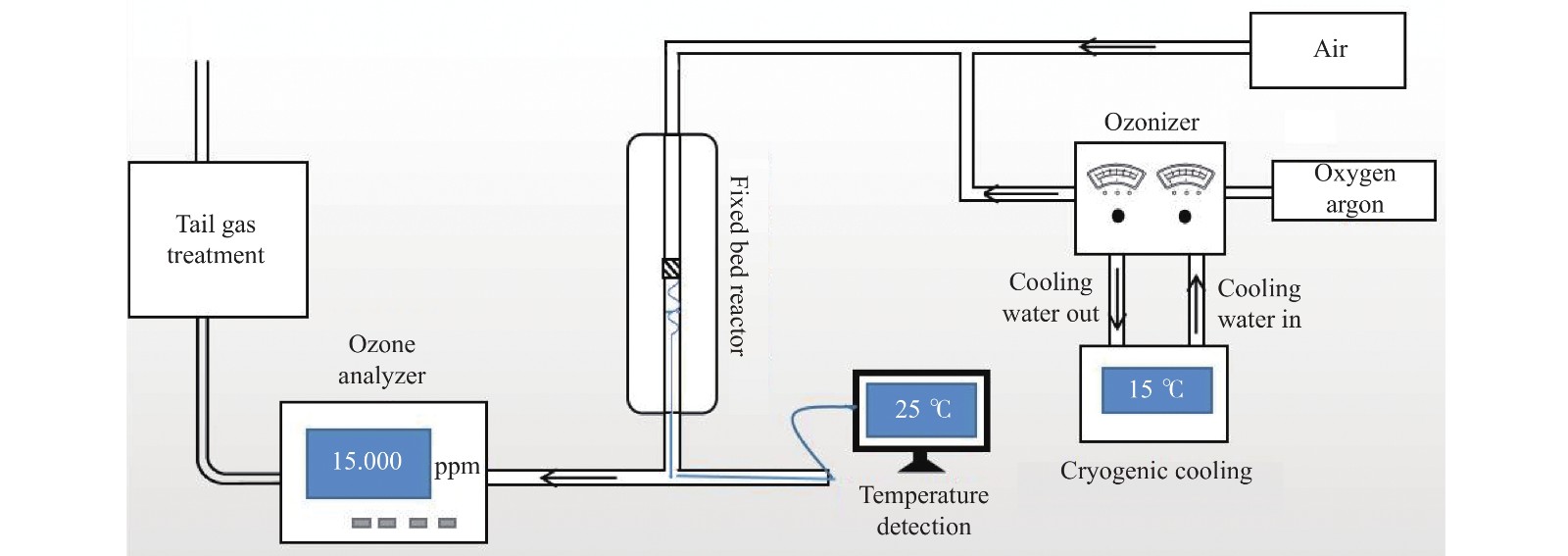
Study on catalytic performance of supported transition metal oxide catalyst for ozone decomposition
-
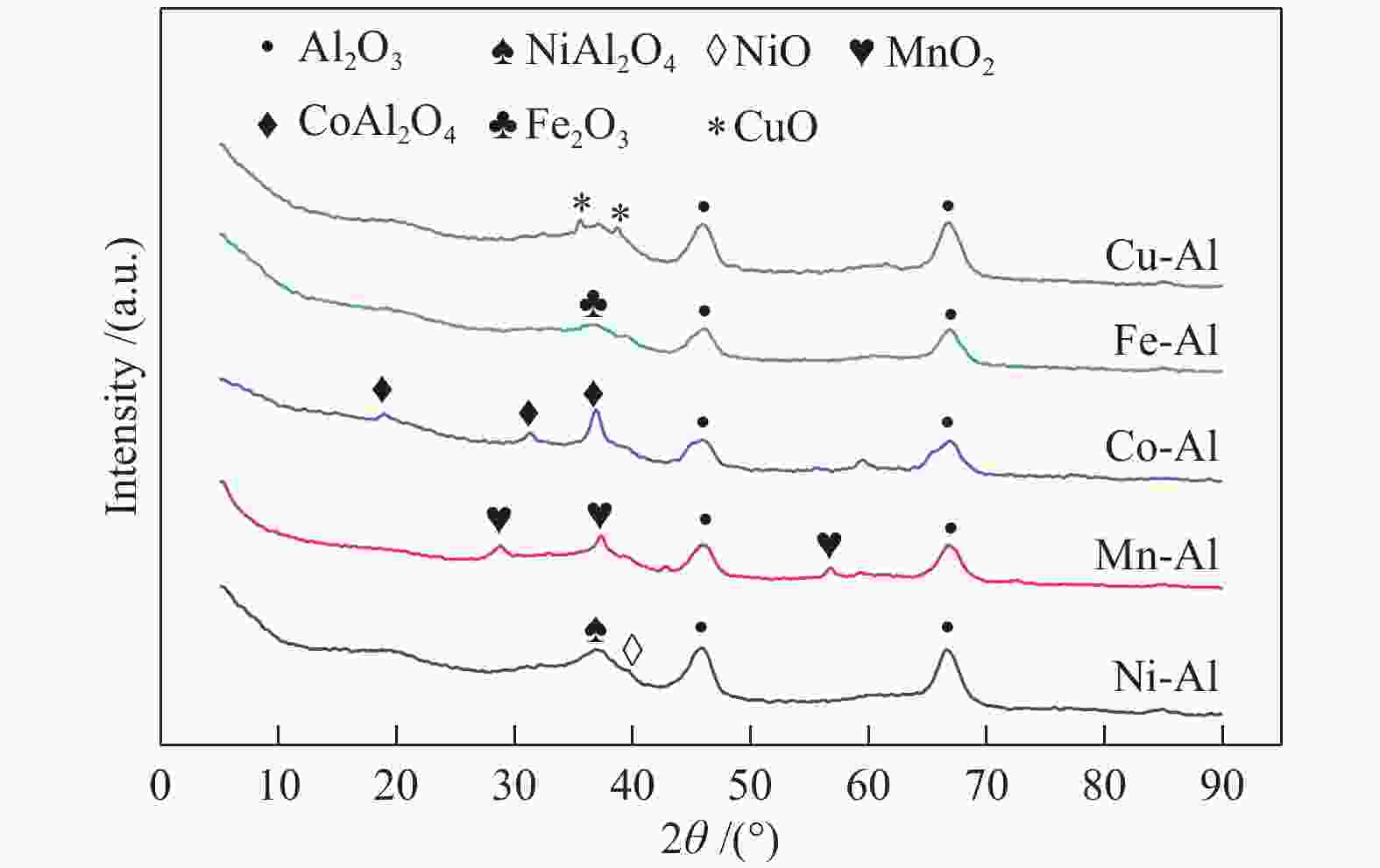
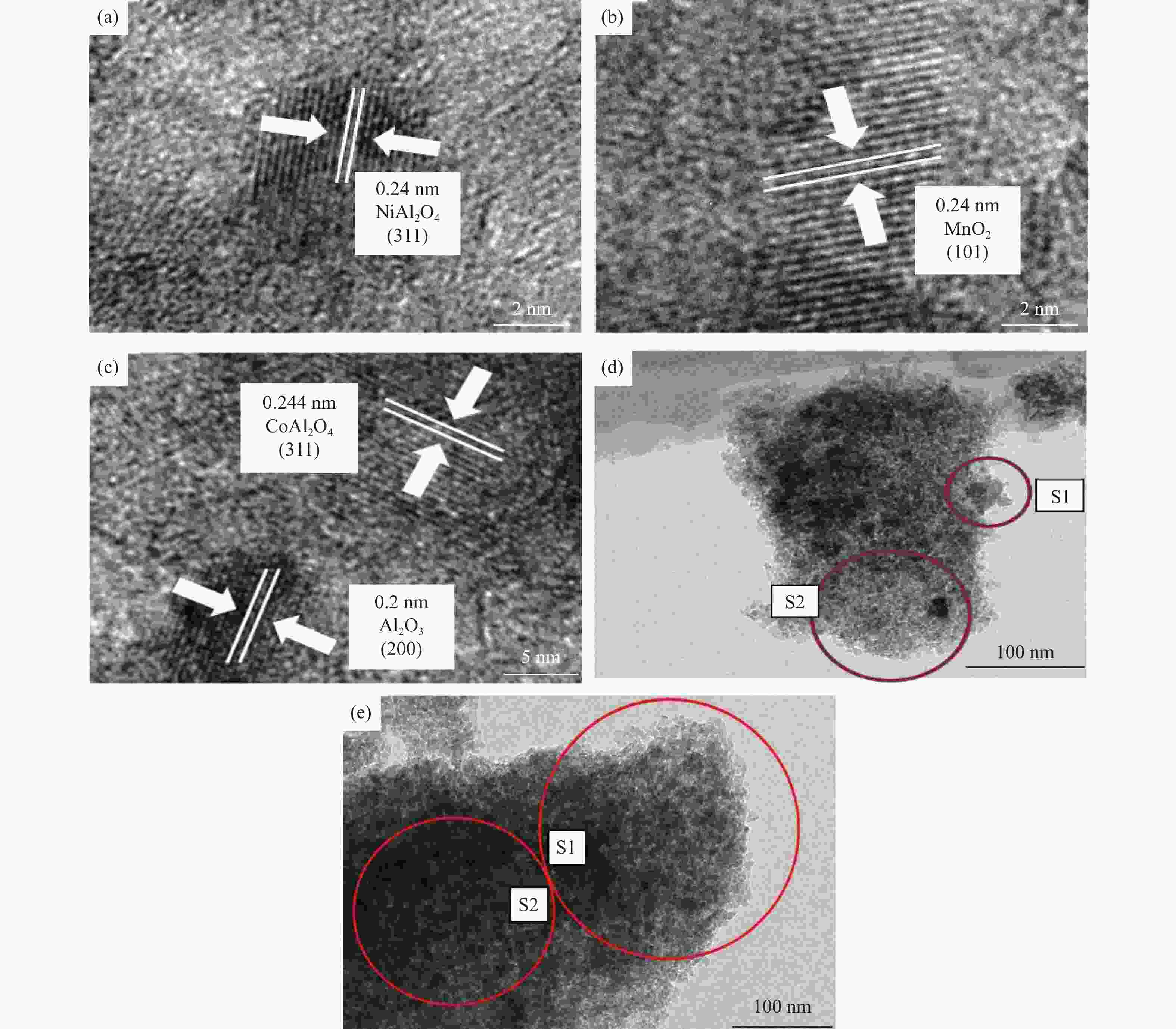
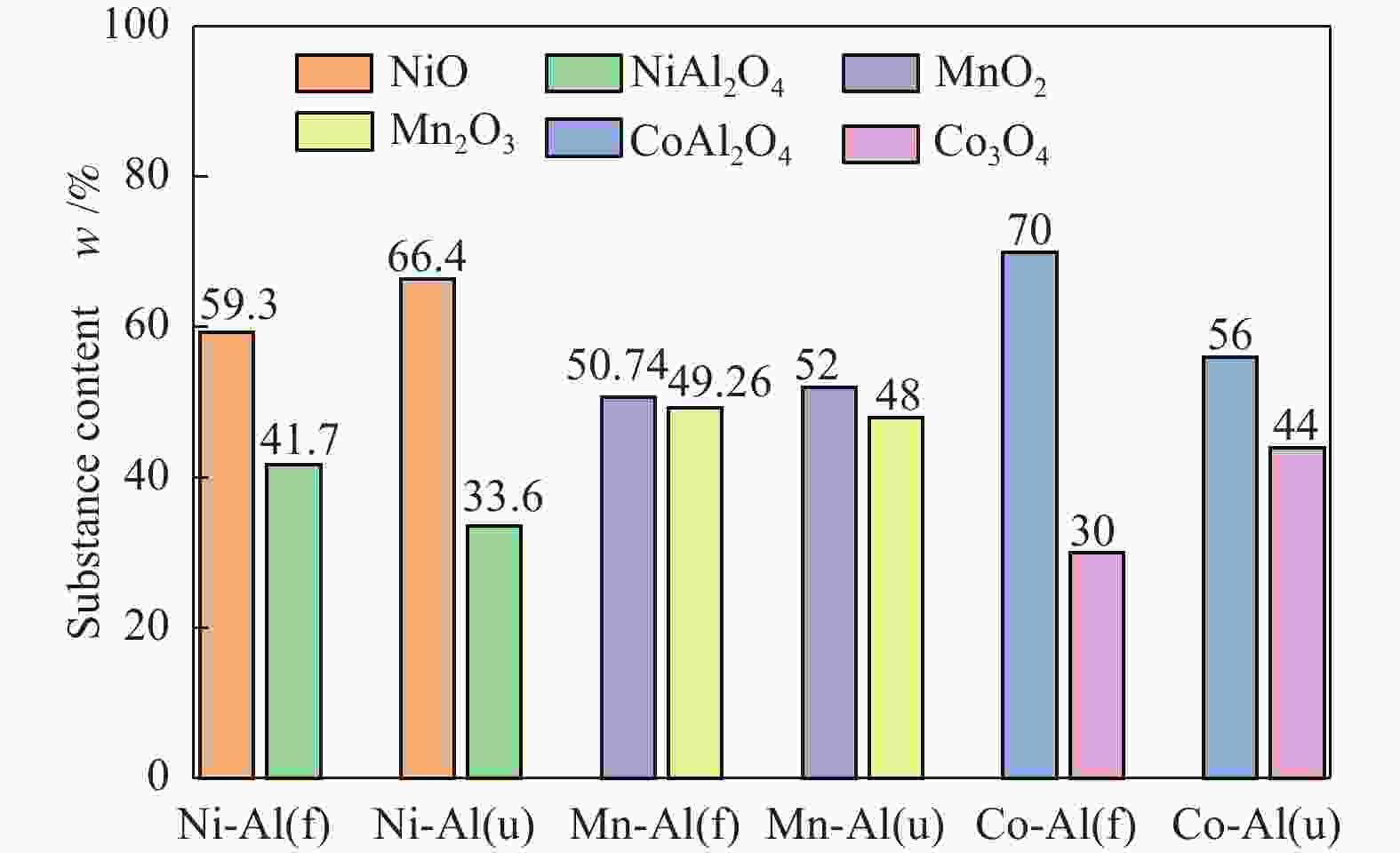
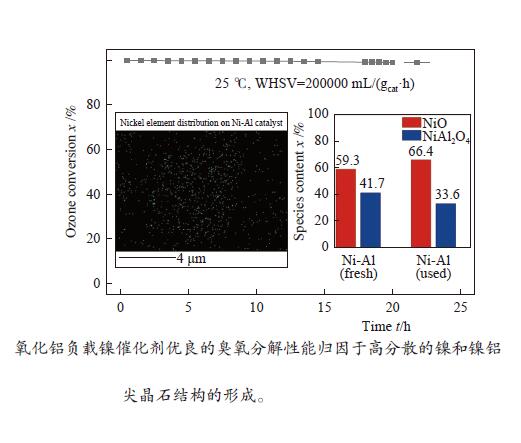
摘要: 通过浸渍法制备了γ-Al2O3负载镍、锰、钴等不同金属氧化物催化剂,在25 ℃、200000 mL/(gcat·h)的空速条件下,研究了其臭氧催化分解性能。结果表明,10%NiO/γ-Al2O3催化剂催化活性最佳,20 h内臭氧转化率高于96%。借助XRD、XPS、TEM、SEM-EDS、H2-TPR等表征手段,揭示出在NiO/γ-Al2O3催化剂表面形成的镍铝尖晶石结构可能是其优良臭氧分解性能的内在原因。此外,揭示出在不同过渡金属氧化物负载型催化剂上臭氧分解机理不同,相关研究为镍、锰等过渡金属氧化物催化剂催化分解臭氧的反应机理提供了新思路,并为开发高效的臭氧分解催化剂提供了指导。Abstract: In this paper, γ-Al2O3 supported nickel, manganese, cobalt, and other metal oxide catalysts were prepared by the impregnation method respectively, and its ozone catalytic decomposition performance was studied at 25 ℃ under a WHSV of 200000 mL/(gcat·h). The results showed that 10% NiO/γ-Al2O3 catalyst demonstrates superior catalytic activity, and the ozone conversion rate is higher than 96% within 20 h. According to the characterizations of XRD, XPS, TEM, SEM-EDS and H2-TPR, its excellent ozone may be attributed to the formation of NiAl2O4 spinel on the surface of NiO/γ-Al2O3 catalyst. Furthermore, the mechanism of ozone decomposition on different transition metal oxide catalysts is divergent. The related study sheds new light on the reaction mechanism of ozone catalytic decomposition over transition metal oxides such as nickel and manganese, it also provides the guidelines for the development of efficient ozone decomposition catalysts.
-
Key words:
- ozone /
- catalytic decomposition /
- catalyst /
- metal oxide
-
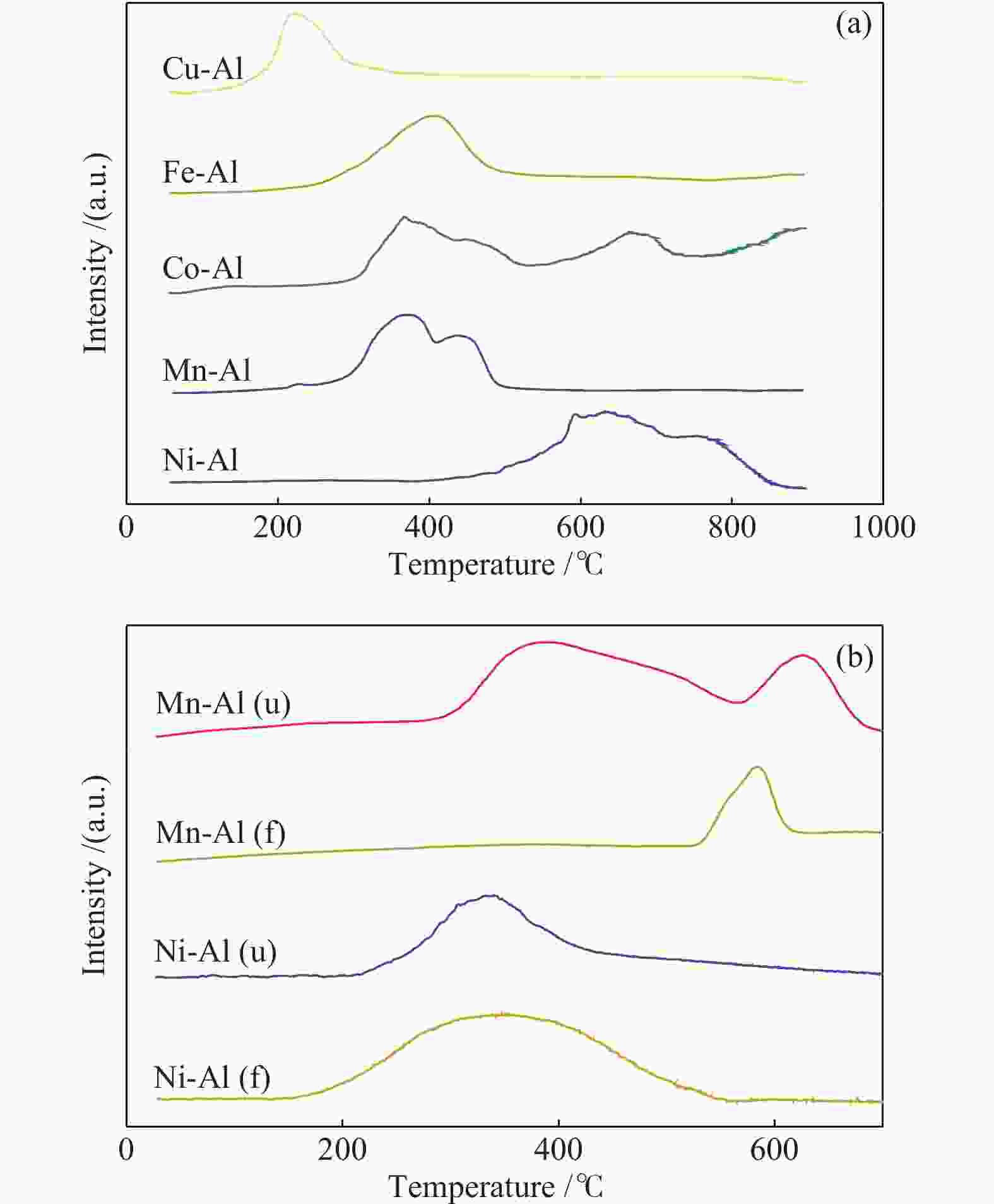
表 1 不同催化剂的理化结构参数
Table 1 Texture properties of different catalysts
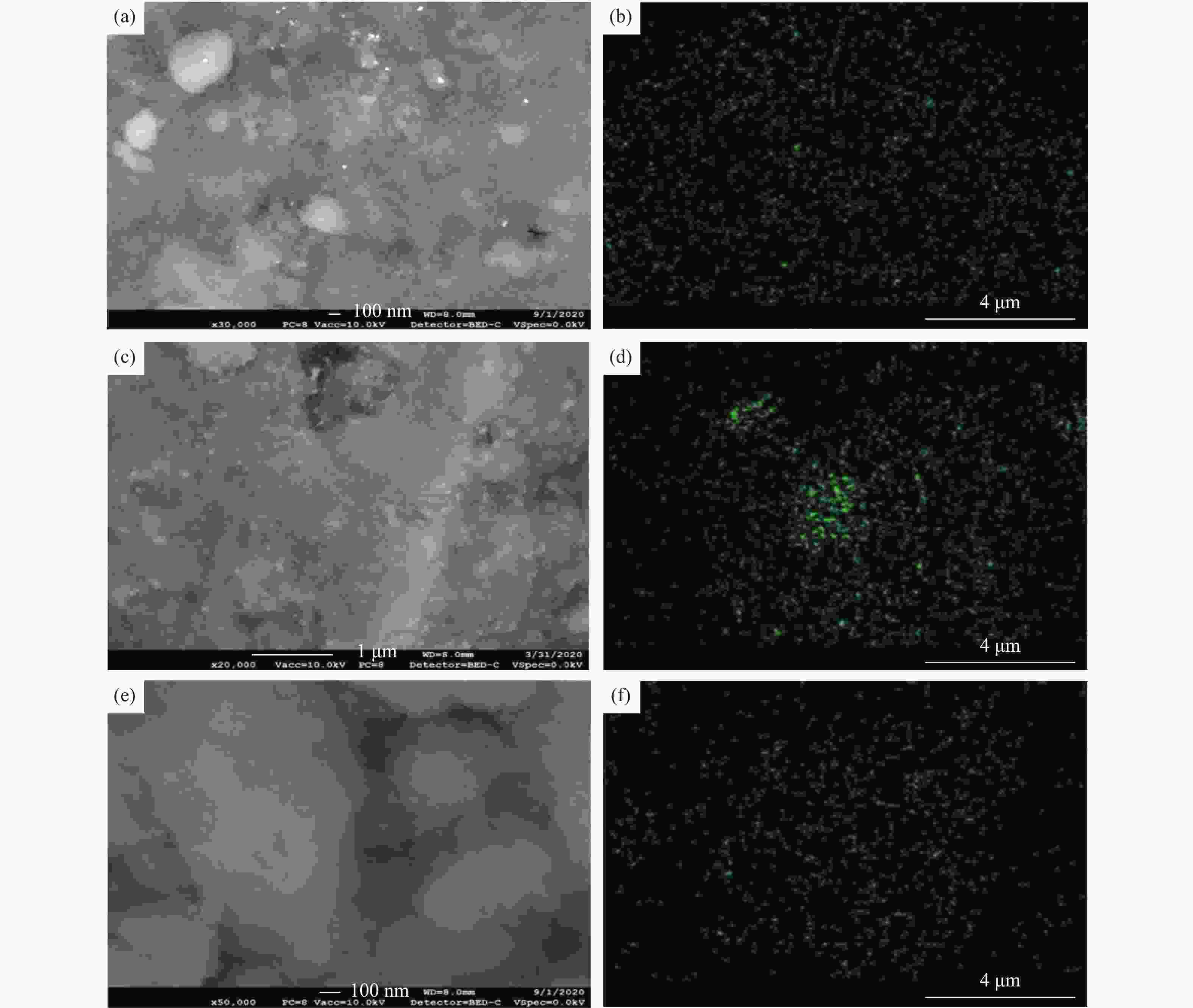
Sample SBET/(m2·g−1) Pore volume/(cm3·g−1) Pore diameter/nm γ-Al2O3 202 0.334 4.34 Ni-Al 175 0.293 4.34 Mn-Al 177 0.292 4.34 Co-Al 168 0.268 4.33 Fe-Al 180 0.27 4.33 Cu-Al 175 0.27 4.33 表 2 钴氧化物负载型催化剂的元素分析
Table 2 Elemental distributions over the Co-Al catalyst
Element Weight/% Atomic/% S1 S2 S1 S2 O 40.46 33.02 53.89 50.07 Al 57.40 45.88 45.34 41.25 Co 2.14 21.10 0.77 8.68 表 3 镍氧化物负载型催化剂的元素分析
Table 3 Elemental distributions over the Ni-Al catalyst
Element Weight/% Atomic/% S1 S2 S1 S2 O 46.61 41.89 61.37 56.48 Al 46.15 51.31 36.03 41.02 Ni 7.25 6.79 2.6 2.5 -
[1] BERNSTEIN J A, ALEXIS N, BACCHUS H. The health effects of nonindustrial indoor air pollution[J]. J Allergy Clin, Immunol,2008,121:585−591. doi: 10.1016/j.jaci.2007.10.045 [2] BERNSTEIN J A, ALEXIS N, BARNES C. Health effects of air pollution[J]. J Allergy Clin, Immunol,2004,114:1116−1123. doi: 10.1016/j.jaci.2004.08.030 [3] ATKINSON R. Atmospheric chemistiy of VOCs and NOx[J]. Atmos Environ,2000,34:2063−2101. doi: 10.1016/S1352-2310(99)00460-4 [4] SAAKE B, LEHNEN R, SCHMEKAL E. Bleaching of formacell pulp from aspen wood with ozone and preacetic acid in organic solvents[J]. Holzforschung,1998,52:643−650. doi: 10.1515/hfsg.1998.52.6.643 [5] CAMEL V, BERMOND A. The use of ozone processes in drinking water treatment[J]. Wat Res,1998,32:3208−3222. doi: 10.1016/S0043-1354(98)00130-4 [6] ZEYNEP B, ANNEL K G, SEYDIM A C. Use of ozone in the food industry[J]. Lwt-Food Sci Technol,2004,37:453−460. doi: 10.1016/j.lwt.2003.10.014 [7] CHANG L T, HONG G B, WENG S P. Indoor ozone levels, houseplants and peak expiratory flow rates among healthy adults in Taipei, Taiwan[J]. Environ Int,2019,122:231−236. doi: 10.1016/j.envint.2018.11.010 [8] WESCHLER C J. Chemistry in indoor environments: 20 years of research[J]. Indoor Air,2011,3:205−218. [9] WESCHLER C J. Ozone in indoor environments: Concentration and chemistry[J]. Indoor Air,2000,10:269−288. doi: 10.1034/j.1600-0668.2000.010004269.x [10] 谢甫钦柯 M A. 臭氧化法水处理工艺学[M]. 北京: 清华大学出版社, 1987.CHEVCHENKO M A. Ozonation Water Treatment Technology [M]. Beijing: Tsinghua University Press, 1987. [11] AXWORTHY A E, BENSON S W. Mechanism of gas phase decomposition of ozone. Thermal and photochemical reactions[J]. Ozone Chem Technol,1959,21:388−397. [12] WANG Y, ARANDIYAN H, SCOTT J. Recent advances in ordered meso/macroporous metal oxides for heterogeneous catalysis: A review[J]. J Mater Chem A,2017,19:8825−8846. [13] CAO S, TAO F F, TANG Y. Size- and shape-dependent catalytic performances of oxidation and reduction reactions on nanocatalysts[J]. Chem Soc Rev,2016,17:4747−65. [14] CHEN Z, JIAO Z, PAN D. Recent advances in manganese oxide nanocrystals: Fabrication, characterization, and microstructure[J]. Chem Rev,2012,7:3833−55. [15] SHI J. On the synergetic catalytic effect in heterogeneous nanocomposite catalysts[J]. Chem Rev,2013,3:2139−2181. [16] STOERZINGER K A, RISCH M, HAN B. Recent insights into manganese oxides in catalyzing oxygen reduction kinetics[J]. ACS Catal,2015,10:6021−6031. [17] TANG W, LIU G, LI D. Design and synthesis of porous non-noble metal oxides for catalytic removal of VOCs[J]. Sci Sin Chim,2015,9:1359−1366. [18] YU Q W, PAN H, ZHAO M, LIU Z M, WANG J L, CHEN Y Q, GONG M C. Influence of calcination temperature on the performance of Pd-Mn/SiO2-Al2O3 catalysts for ozone decomposition[J]. J Hazard Mater,2009,172:631−634. doi: 10.1016/j.jhazmat.2009.07.040 [19] DHANDAPANI, RAGHAVAN S. Probabilistic and topological methods in computational geometry[D]. New York: New York University, 2010. [20] IMAMURA S, IKEBATA M, ITO T. Decomposition of ozone on a silver catalyst[J]. Ind Eng Chem Res,1991,30(1):217−221. doi: 10.1021/ie00049a033 [21] RADHAKRISHNAN R, OYAMA S T. Electron transfer effects in ozone decomposition on supported manganese oxide[J]. J Phys Chem B,2001,105(19):4245−4253. doi: 10.1021/jp003246z [22] GONG S Y, CHEN J Y, WU X F. In-situ synthesis of Cu2O/reduced graphene oxide composite as effective catalyst for ozone decomposition[J]. Catal Commun,2018,106:25−29. doi: 10.1016/j.catcom.2017.12.003 [23] RADHAKRISHNAN R, OYAMA S T. Ozone decomposition over manganese oxide supported on ZrO2 and TiO2: A kinetic study using in situ laser raman spectroscopy[J]. J Catal,2001,199(2):282−290. doi: 10.1006/jcat.2001.3167 [24] DAN C, POPOVICI E J, IMRE F, INDREA E, MARGINEAN P, SILAGHI-DUMITRESCU I. Studies on some ozone decomposition catalysts based on nickel oxide[J]. Stud Univ Babes-Bolyai Chem,2007,52(1):91−95. [25] CAO R R, ZHANG P Y, LIU Y. Ammonium-treated birnessite-type MnO2 to increase oxygen vacancies and surface acidity for stably decomposing ozone in humid condition[J]. Appl Surf Sci,2019,495:143607. doi: 10.1016/j.apsusc.2019.143607 [26] MARZIEHOSSADAT S Y, OMMOLBANIN A S, MUSTAPHA A, MARIA C. A novel synthesis of NiAl2O4 spinel from a Ni-Al mixed-metal alkoxide as a highly effcient catalyst for hydrogen production by glycero lsteam reforming[J]. Appl Catal B: Environ,2020,265:118535. doi: 10.1016/j.apcatb.2019.118535 [27] YANG Y J. The effffect of tungsten doping on the catalytic activity of α-MnO2 nanomaterial for ozone decomposition under humid condition[J]. Appl Catal A: Gen,2018,562:132−141. doi: 10.1016/j.apcata.2018.06.006 [28] ZHANG G F. Facile fabrication of Al2O3-doped Co3O4/graphene nanocomposites for high performance asymmetric supercapacitors[J]. Appl Surf Sci,2019,493:55−62. doi: 10.1016/j.apsusc.2019.06.288 [29] PETZOLD F G, JASINSKI J, CLARK E L, KIM J H, ABSHER J, TOUFAR H, SUNKARA M K. Nickel supported on zinc oxide nanowires as advanced hydrodesulfurization catalysts[J]. Catal Today,2012,198(1):219−227. doi: 10.1016/j.cattod.2012.05.030 [30] GOICOECHEA S, KRALEVA E, SOKOLOV S, SCHNEIDER M, POHI M M, KOCKMANN N, EHRICH H. Support effect on structure and performance of Co and Ni catalysts for steam reforming of acetic acid[J]. Appl Catal A: Gen,2016,514:182−191. doi: 10.1016/j.apcata.2015.12.025 [31] MISHRA T, MOHAPATRA P, PARIDA K M. Characterisation and catalytic evaluation of iron–manganese mixed oxide pillared clay for VOC decomposition reaction[J]. Appl Catal B: Environ,2008,79(3):279−285. doi: 10.1016/j.apcatb.2007.10.030 [32] ANDRÉ V H S. Palladium and nickel supported on Fe3O4 as catalysts for glycerol aqueous-phase hydrogenolysis and reforming[J]. Appl Phys A,2017,548:179−190. [33] FEDOROV A V, KUKUSHKIN R G, YELETSKY P M, BULAVCHENKO O A, YAKOVLEV V A. Temperature-programmed reduction of model CuO, NiO and mixed CuO-NiO catalysts with hydrogen[J]. J. Alloys Compd,2020,844:156135. doi: 10.1016/j.jallcom.2020.156135 [34] CHEN J, CHEN X, CHEN X. Homogeneous introduction of CeOy into MnOx-based catalyst for oxidation of aromatic VOCs[J]. Appl Catal B: Environ,2018,224:825−835. doi: 10.1016/j.apcatb.2017.11.036 [35] JIA J B, ZHANG P Y, CHEN L. The effect of morphology of α-MnO2 on catalytic decomposition of gaseous ozone[J]. Catal Sci Technol,2016,15(6):5841. [36] GAO Y, WANG S, LV L R, LI D Y, YUE X, WANG S D. Insights into the behaviors of the catalytic combustion of propane over spinel catalysts[J]. Catal Lett,2020,150:3617−3625. doi: 10.1007/s10562-020-03239-3 [37] ERTL G, HIERL R, KNÖZINGER H, THIELE N, URBACH H P. XPS study of copper aluminate catalysts[J]. Appl Sci,1980,5(1):49−64. [38] KASZTELAN S, GRIMBLOT J, BONNELLE J P, PAYEN E, TOULHOAT H, JACQUIN Y. Preparation of Co-Mo-γ-Al2O3 and Ni-Mo-γ-Al2O3 catalysts by phregulation of molybdenum solution. characterization of supported species and hydrogenation activities[J]. Appl Catal,1983,7(1):91−112. doi: 10.1016/0166-9834(83)80241-3 [39] ANSELL R O, DICKINSON T, POVEY A F. An X-ray photo-electron spectroscopic study of the films on coloured stainless steel and coloured ‘Nilomag’ alloy 771[J]. Corros Sci,1978,18(3):245−256. doi: 10.1016/S0010-938X(78)80021-3 [40] CARVER J C, SCHWEITZER G K. Use of X-ray photoelectron spectroscopy to study bonding in Cr, Mn, Fe, and Co compounds[J]. J Chem Phys,1972,57:973. doi: 10.1063/1.1678348 [41] MCINTYRE N S, JOHNSTON D D, COATSWORTH L L, DAVIDSON R D, BROWN J R. X-ray photoelectron spectroscopic studies of thin film oxides of cobalt and molybdenum[J]. J Chem Phys,1990,15(4):265−272. [42] OKAMOTO Y, IMANAKA T, TERANISHI S. Surface structure of CoO-MoO3-Al2O3 catalysts studied by X-ray photoelectron spectroscopy[J]. J Catal,1980,65(2):448−460. doi: 10.1016/0021-9517(80)90322-X -





 下载:
下载: