Selective breaking of C−O bonds in hydrodeoxygenation of 4-methylphenol over CoMoS/ZrO2
-
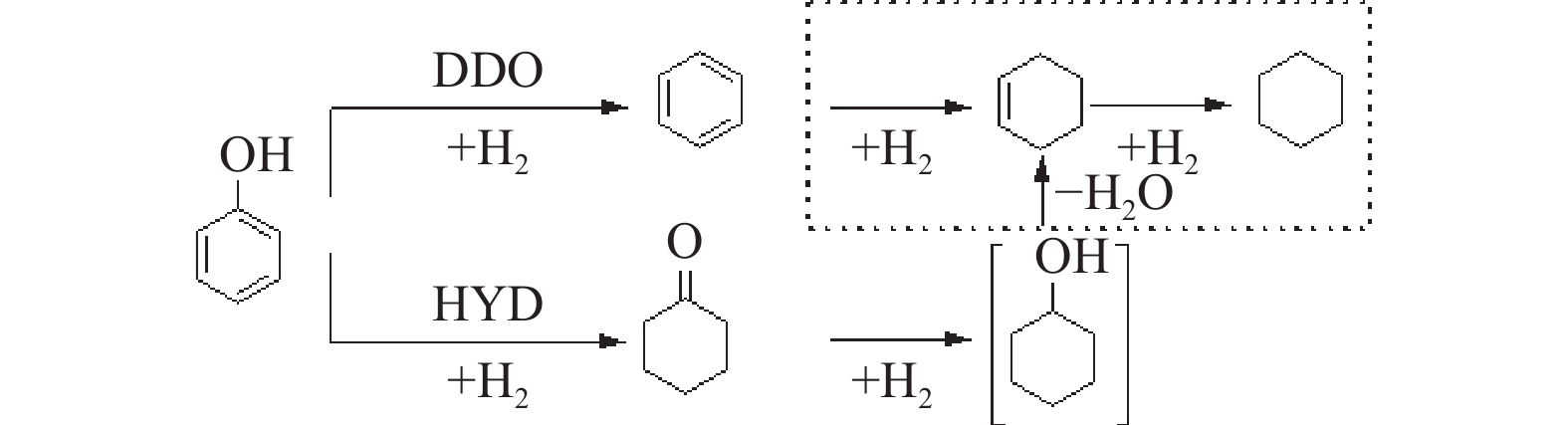
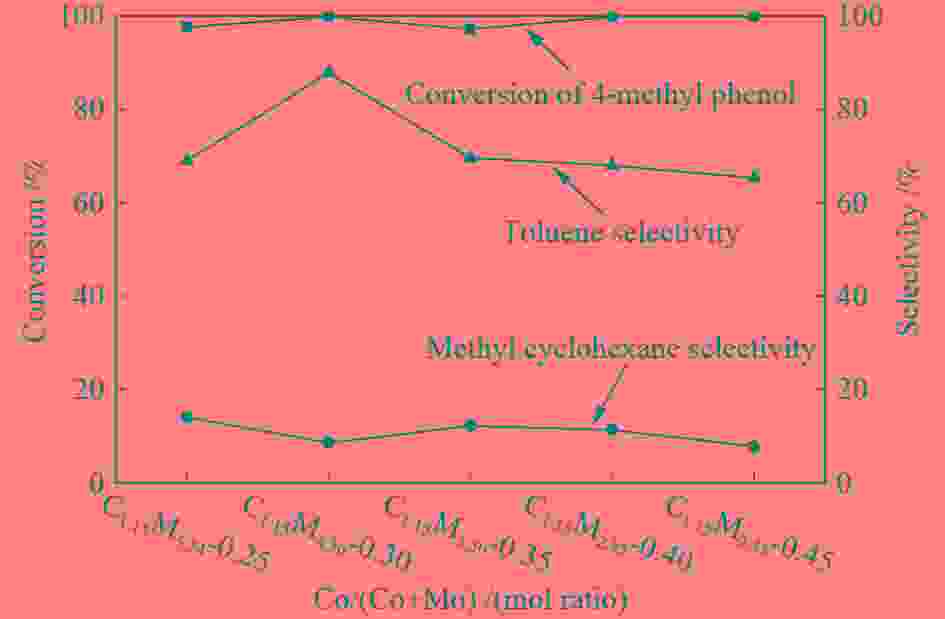
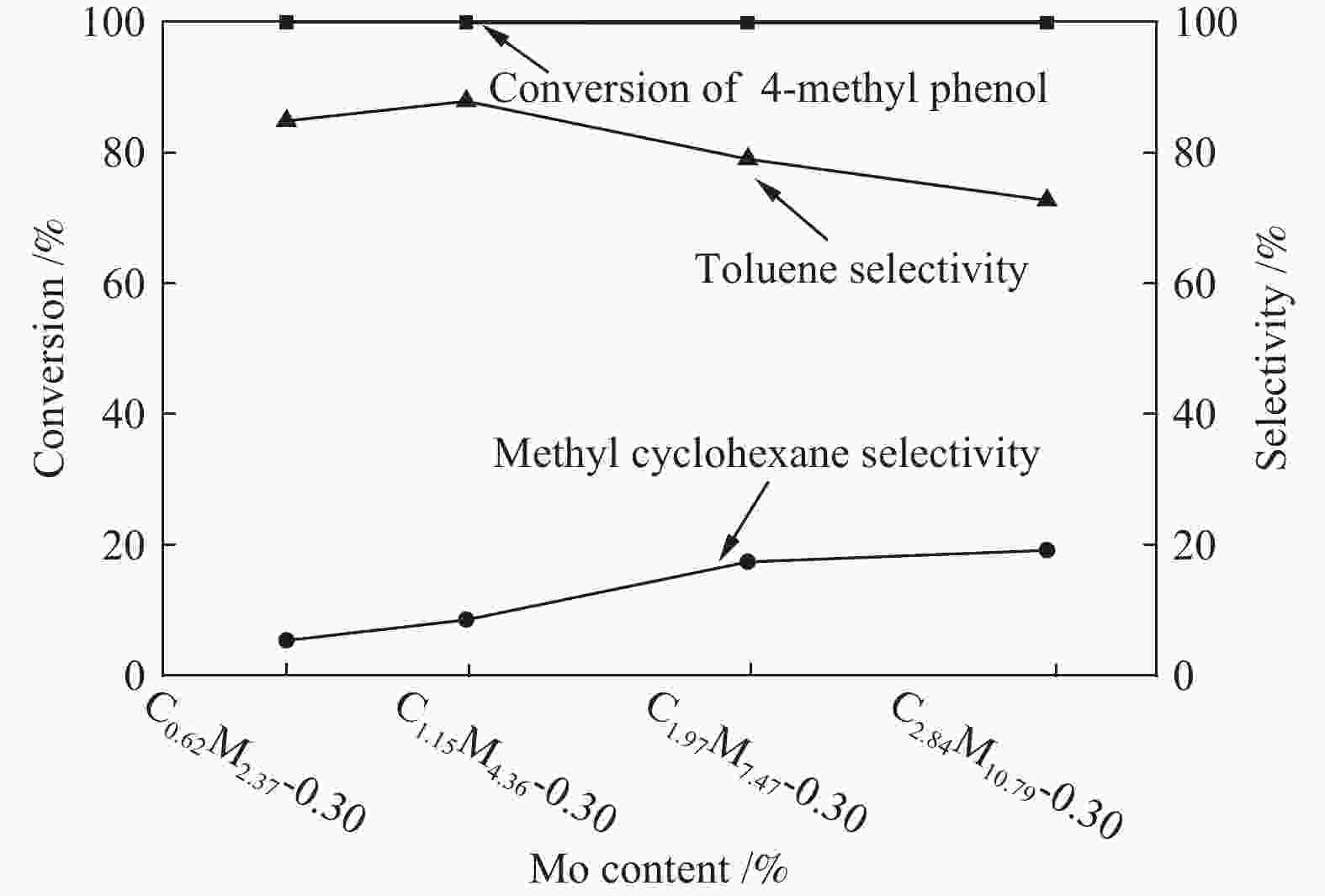
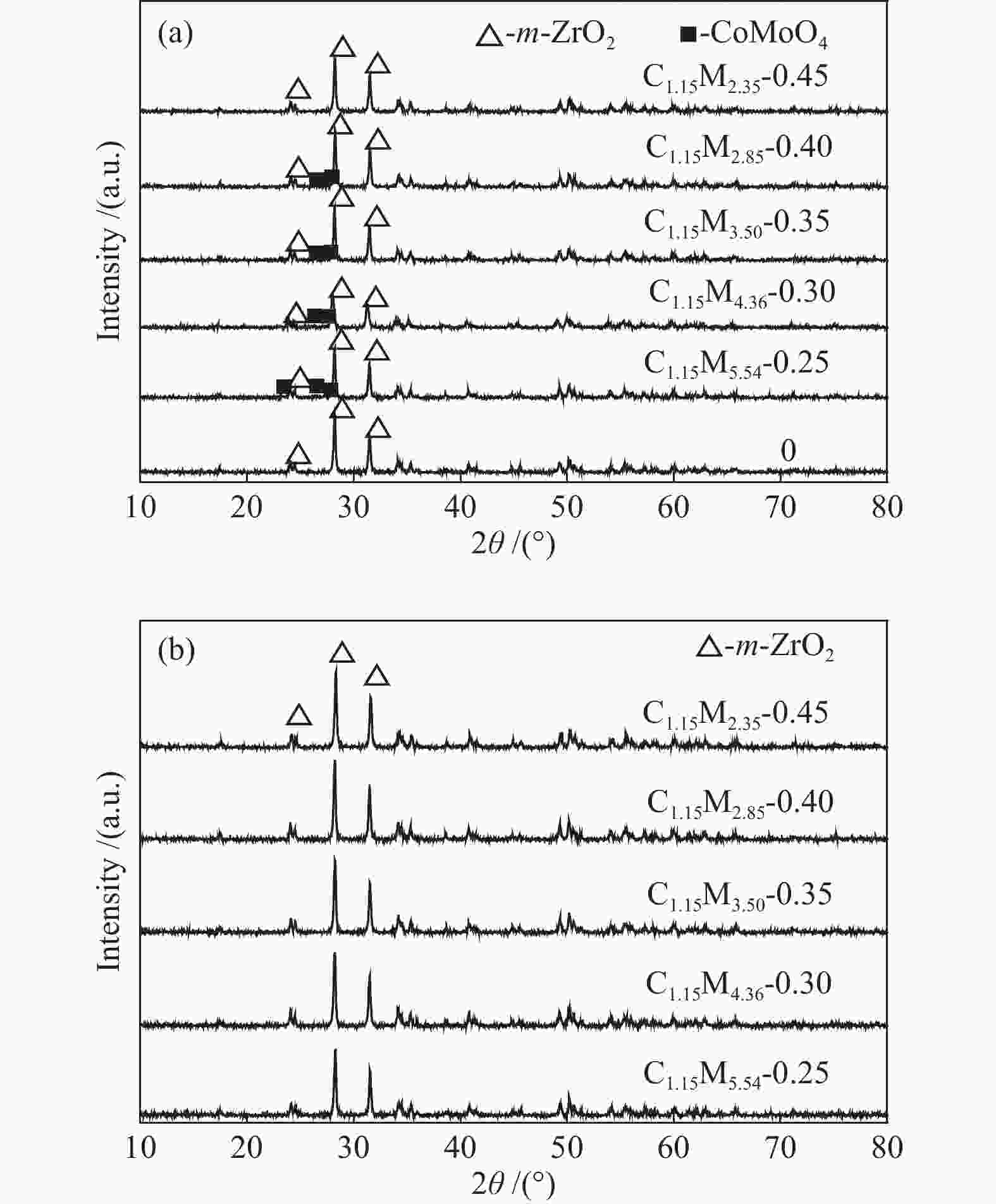
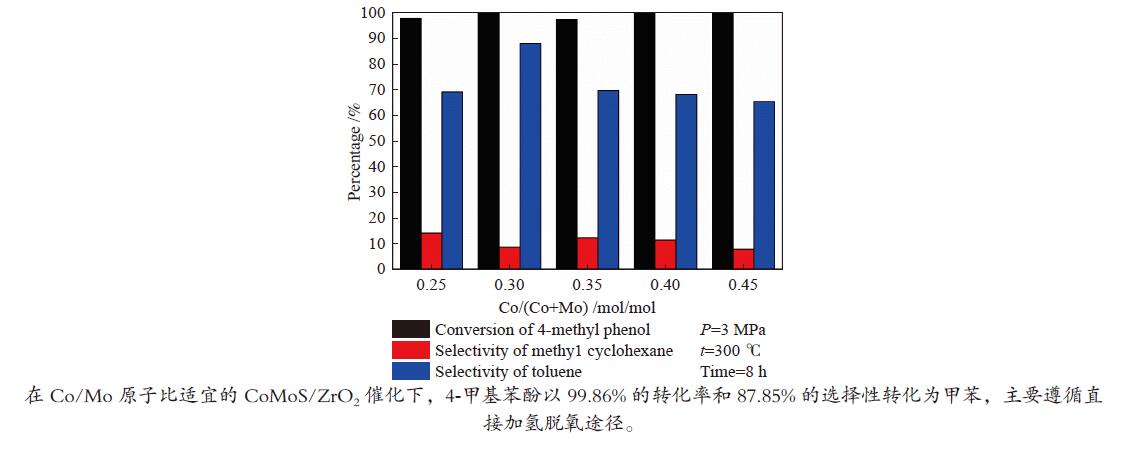
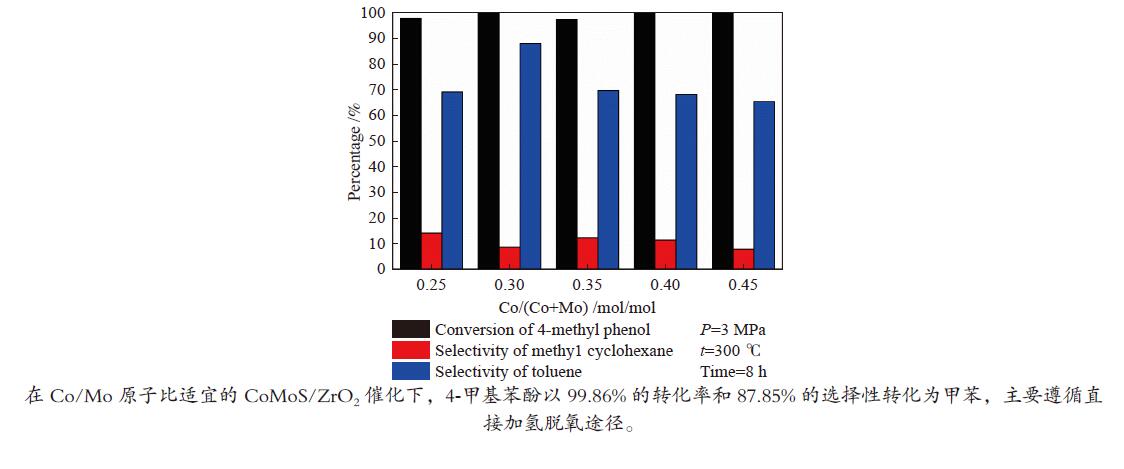
摘要: 采用饱和浸渍法制备了不同Co-Mo原子比(0.25,0.30,0.35,0.40,0.45)和Co-Mo负载量(2.35%,4.36%,7.48%和10.79%)的CoMoS/ZrO2催化剂。用X射线衍射(XRD)、程序升温还原(H2-TPR)、氮气吸附和X射线光电子能谱(XPS)对催化剂进行了表征。以4-甲基苯酚为模型化合物进行加氢脱氧反应。结果表明,当Co-Mo(Co/Co+Mo)原子比为0.30,Mo负载量为4.36%时,催化加氢活性最好,4-甲基苯酚的转化率可达99.86%,直接加氢脱氧产物甲苯的选择性达到87.85%,较高程度保持了芳环。CoMoO4的产生不利于甲苯的生成。Co-Mo和ZrO2之间需要适当的相互作用。Abstract: CoMoS/ZrO2 catalysts with different Co-Mo atomic ratios (0.25, 0.30, 0.35, 0.40 and 0.45) and Co-Mo loading amounts (2.35%, 4.36%, 7.48% and 10.79%) were prepared by incipient wetness impregnation. These catalysts were characterized by X-ray diffraction (XRD), temperature-programmed reduction (H2-TPR), nitrogen adsorption/desorption and X-ray photoelectron spectroscopy (XPS). 4-methylphenol was used as model compound for hydrodeoxygenation reaction. The result showed that when the atomic ratio of Co-Mo (Co/Co + Mo) was 0.30 and the Mo loading amount was 4.36%, the highest hydrogenation activity was observed. The conversion of 4-methylphenol was up to 99.86% and the selectivity of main product toluene reached to 87.85%. The formation of CoMoO4 was unfavourable to the formation of toluene. An appropriate interaction between Co-Mo and ZrO2 was required.
-
Key words:
- hydrodeoxygenation /
- phenols /
- 4-methylphenol /
- transition metal sulfides /
- zirconia
-
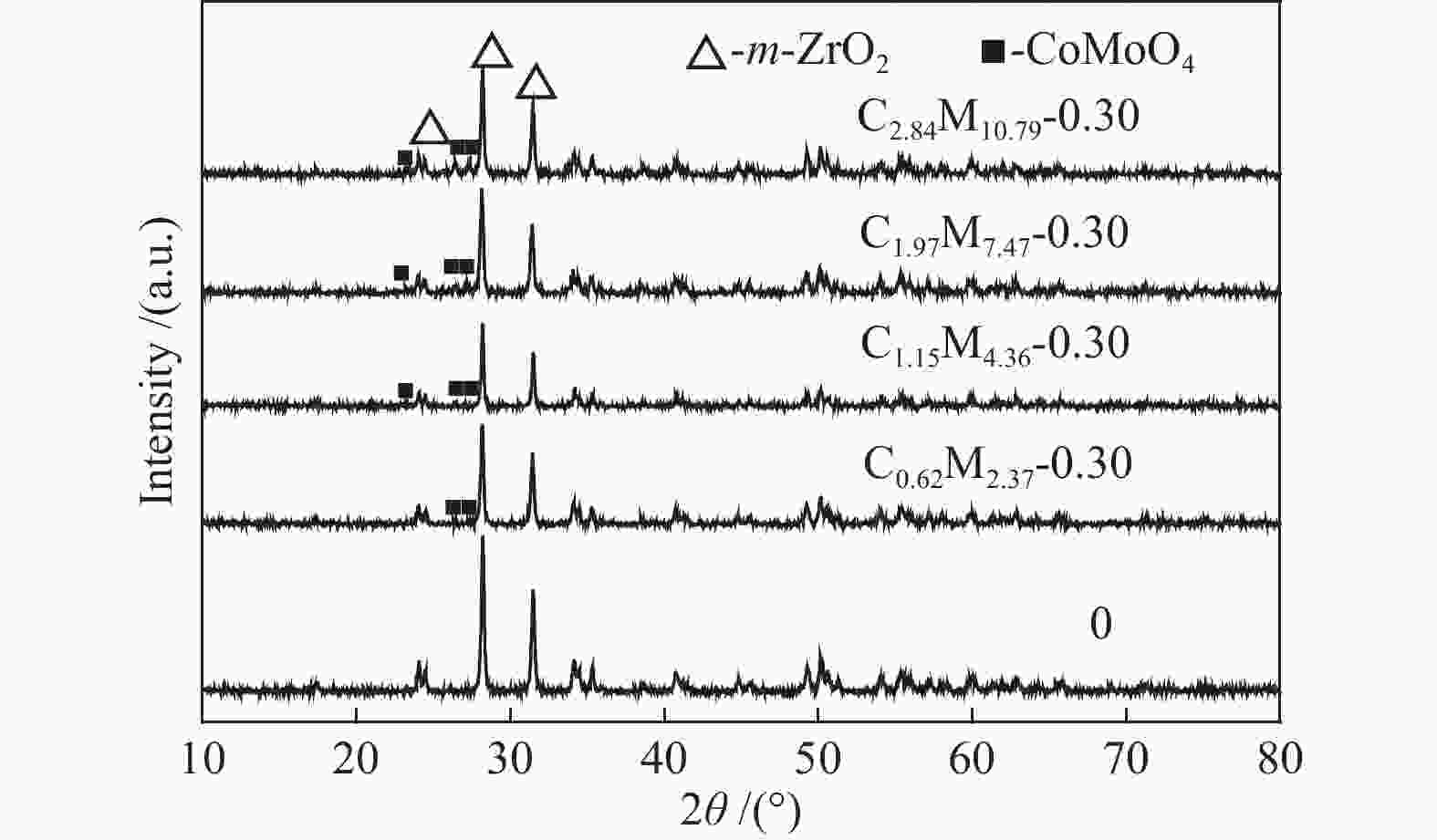
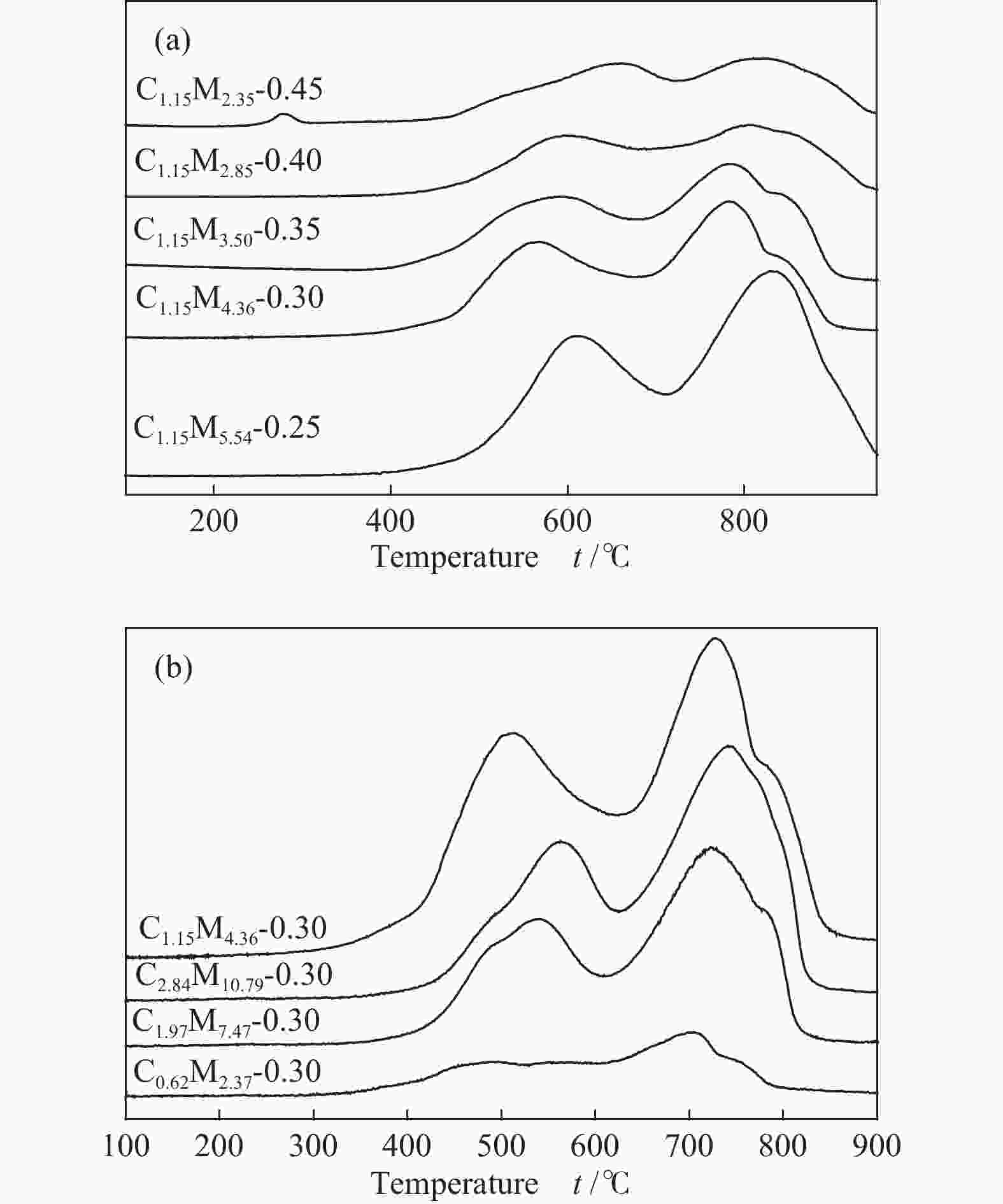
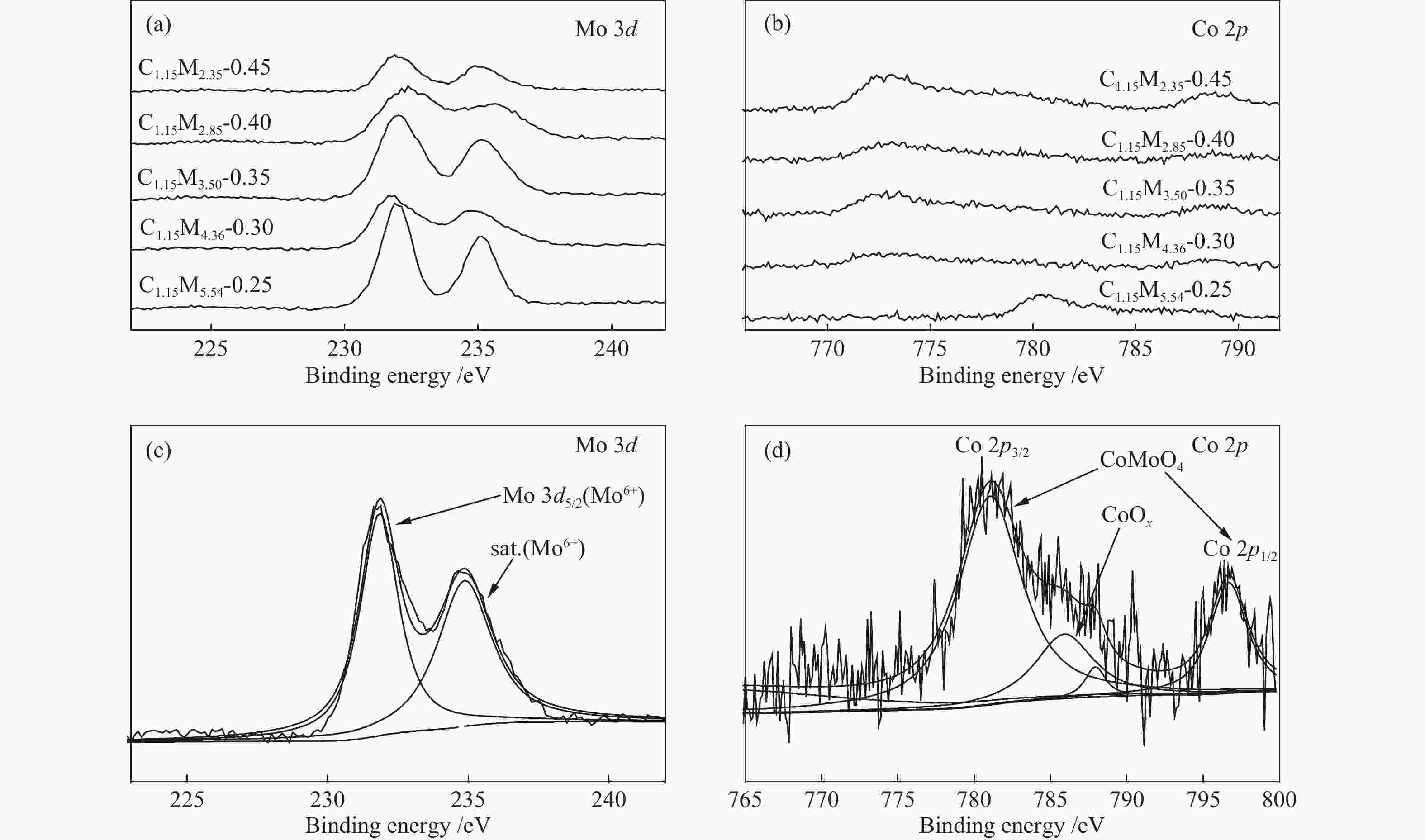
Table 1 CoMo/ZrO2 catalysts with different Co-Mo atomic ratios
Catalyst Cobalt
content w/%Molybdenum
conten w/%Co-Mo atomic
ratioCo/(Co+Mo)
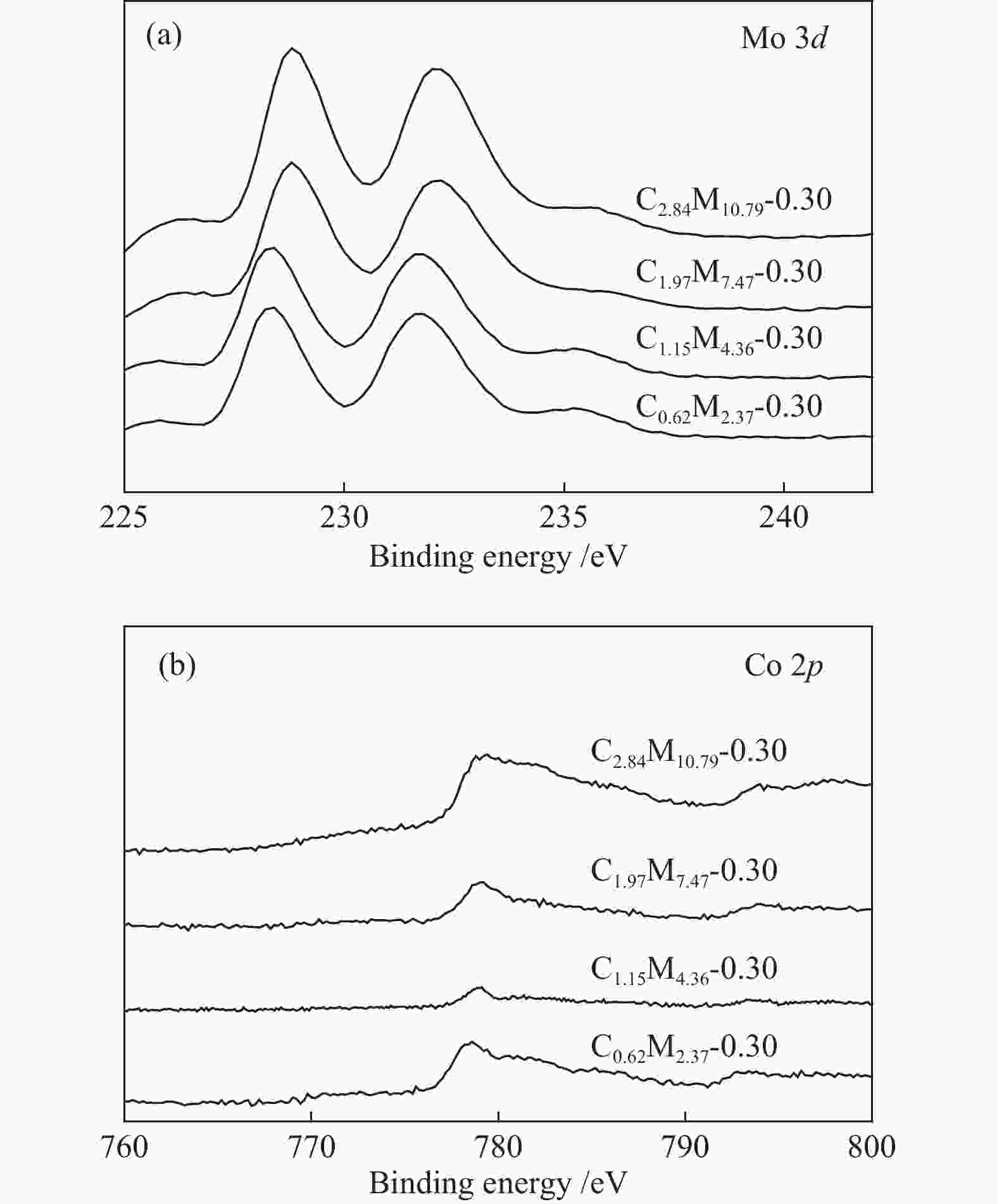
(mol ratio)C1.15M5.54-0.25 1.15 5.54 0.25 C1.15M4.36-0.30 1.15 4.36 0.30 C1.15M3.50-0.35 1.15 3.50 0.35 C1.15M2.85-0.40 1.15 2.85 0.40 C1.15M2.35-0.45 1.15 2.35 0.45 Table 2 CoMo/ZrO2 catalysts with different Co-Mo loading
Catalyst Cobalt
content w/%Molybdenum
content w/%Co-Mo atomic
ratioCo/(Co+Mo)
(mol ratio)C0.62M2.37-0.30 0.62 2.37 0.30 C1.15M4.36-0.30 1.15 4.36 0.30 C1.97M7.47-0.30 1.97 7.47 0.30 C2.84M10.79-0.30 2.84 10.79 0.30 -
[1] SAIDI M, SAMIMI F, KARIMIPOURFARD D. Upgrading of lignin-derived bio-oils by catalytic hydrodeoxygenation[J]. Energy Environ Sci,2014,7:103−129. doi: 10.1039/C3EE43081B [2] FURIMSKY E. Catalytic hydrodeoxygenation[J]. Appl Catal A: Gen,2000,199(2):147−190. doi: 10.1016/S0926-860X(99)00555-4 [3] SUN Z, BÁLINT FRIDRICH, SANTI A D. Bright side of lignin depolymerization: Toward new platform chemicals[J]. Chem Rev,2018,118(2):614−678. doi: 10.1021/acs.chemrev.7b00588 [4] WEI T, FANG M, WANG H. Mild hydrotreatment of low temperature coal tar distillate: Product composition[J]. Chem Eng J,2014,236:529−537. doi: 10.1016/j.cej.2013.09.038 [5] LECKEL D. Catalytic hydroprocessing of coal-derived gasification residues to fuel blending stocks: Eeffect of reaction variables and catalyst on hydrodeoxygenation (HDO), hydrodenitrogenation (HDN), and hydrodesulfurization (HDS)[J]. Energy Fuels,2006,20(5):1761−1766. doi: 10.1021/ef060034d [6] WANG H, MALE J, WANG Y. Recent advances in hydrotreating of pyrolysis bio-oil and its oxygen-containing model compounds[J]. ACS Catal,2013,3(5):1047−1070. doi: 10.1021/cs400069z [7] KAN T, WANG H, HE H. Experimental study on two-stage catalytic hydroprocessing of middle-temperature coal tar to clean liquid fuels[J]. Fuel,2011,90(11):3404−3409. doi: 10.1016/j.fuel.2011.06.012 [8] ISLAS C A, SUELVES I, CARTER J F. Pyrolysis-gas chromatography/mass spectrometry of fractions separated from a low-temperature coal tar: An attempt to develop a general method for characterising structures and compositions of heavy hydrocarbon liquids[J]. Rapid Commun Mass Spectrom,2002,16(8):774−84. doi: 10.1002/rcm.638 [9] SHI Q, YAN Y, WU X. Identification of dihydroxy aromatic compounds in a low-temperature pyrolysis coal tar by gas chromatography-mass spectrometry (GC-MS) and fourier transform ion cyclotron resonance mass spectrometry (FT-ICR MS)[J]. Energy Fuels,2010,24(10):5533−5538. doi: 10.1021/ef1007352 [10] SUN M, MA X X, YAO Q X. GC-MS and TG-FTIR study of petroleum ether extract and residue from low temperature coal tar[J]. Energy Fuels,2011,25(3):1140−1145. doi: 10.1021/ef101610z [11] SHI Q, PAN N, LONG H. Characterization of middle-temperature gasification coal tar. part 3: molecular composition of acidic compounds[J]. Energy Fuels,2013,27(1):108−117. doi: 10.1021/ef301431y [12] JONGERIUS A L, JASTRZEBSKI R, BRUIJNINCX P C A. CoMo sulfide-catalyzed hydrodeoxygenation of lignin model compounds: An extended reaction network for the conversion of monomeric and dimeric substrates[J]. J Catal,2012,285(1):315−323. doi: 10.1016/j.jcat.2011.10.006 [13] ZHANG Z, YUE C, HU J. Fabrication of porous MoS2, with controllable morphology and specific surface area for hydrodeoxygenation[J]. Nano,2017,12(9):1750116. doi: 10.1142/S1793292017501168 [14] LIU G, ROBERTSON A W, LI M J. MoS2 monolayer catalyst doped with isolated Co atoms for the hydrodeoxygenation reaction[J]. Nat Chem,2017,9(8):810−816. doi: 10.1038/nchem.2740 [15] SONG W, ZHOU S, HU S, LAI W, LIAN Y J, WANG J, YANG W, WANG M, WANG P, JIANG X. Surface engineering of CoMoS nanosulfide for hydrodeoxygenation of lignin-derived phenols to arenes[J]. ACS Catal,2019,9(1):259−268. doi: 10.1021/acscatal.8b03402 [16] BUI V N, LAURENTI P, DELICHÈRE P. Hydrodeoxygenation of guaiacol: Part II: Support effect for CoMoS catalysts on HDO activity and selectivity[J]. Appl Catal B: Environ,2011,101(3/4):246−255. doi: 10.1016/j.apcatb.2010.10.031 [17] ROMERO Y, RICHARD F, BRUNET S. Hydrodeoxygenation of 2-ethylphenol as a model compound of bio-crude over sulfided Mo-based catalysts: Promoting effect and reaction mechanism[J]. Appl Catal B: Environ,2010,98(3/4):213−223. doi: 10.1016/j.apcatb.2010.05.031 [18] RAHIMPOUR H, SAIDI M, ROSTAMI P. Experimental investigation on upgrading of lignin-derived bio-oils: Kinetic analysis of anisole conversion on sulfided CoMo/Al2O3 catalyst[J]. Int J Chem Kinet,2016,48(11):702−713. doi: 10.1002/kin.21026 [19] AQSHA A, KATTA L, MAHINPEY N. Catalytic hydrodeoxygenation of guaiacol as lignin model component using Ni-Mo/TiO2 and Ni-V/TiO2 catalysts[J]. Catal Lett,2015,145:1351−1363. doi: 10.1007/s10562-015-1530-7 [20] MORTENSEN P M, J GRUNWALDT J, P JENSEN P A, JENSEN A D. Screening of catalysts for hydrodeoxygenation of phenol as a model compound for bio-oil[J]. ACS Catal,2013,3(8):1774−1785. doi: 10.1021/cs400266e [21] GUTIERREZ A, KAILA R K, HONKELA M L. Hydrodeoxygenation of guaiacol on noble metal catalysts[J]. Catal Today,2009,147(3/4):239−246. doi: 10.1016/j.cattod.2008.10.037 [22] TELES C A, RABELO-NETO R C, JACOBS G. Hydrodeoxygenation of phenol over zirconia supported catalysts: The effect of metal type on reaction mechanism and catalyst deactivation[J]. ChemCatChem,2017,9(14). [23] DE SOUZA P M, RABELO-NETO R C, BORGES L E P, JACOBS G, DAVIS B H, GRAHAM U M, RESASCO D E NORONHA F B. Effect of zirconia morphology on hydrodeoxygenation of phenol over Pd/ZrO2[J]. ACS Catal,2015,5(12):7385−7398. doi: 10.1021/acscatal.5b01501 [24] FERRARI M, DELMON B, GRANGE P. Influence of the active phase loading in carbon supported molybdenum-cobalt catalysts for hydrodeoxygenation reactions[J]. Microporous Mesoporous Mater,2002,56(3):279−290. doi: 10.1016/S1387-1811(02)00492-4 [25] MAITY S K, FLORES G A, ANCHEYTA J. Effect of preparation methods and content of phosphorus on hydrotreating activity[J]. Catal Today,2008,130(2/4):374−381. doi: 10.1016/j.cattod.2007.10.100 [26] YANG Y, GILBERT A, XU C. Hydrodeoxygenation of bio-crude in supercritical hexane with sulfided CoMo and CoMoP catalysts supported on MgO: A model compound study using phenol[J]. Appl Catal A: Gen,2009,360(2):242−249. doi: 10.1016/j.apcata.2009.03.027 -




 下载:
下载: