Solvothermal synthesis of TiO2@MIL-101(Cr) for efficient photocatalytic fuel denitrification
-
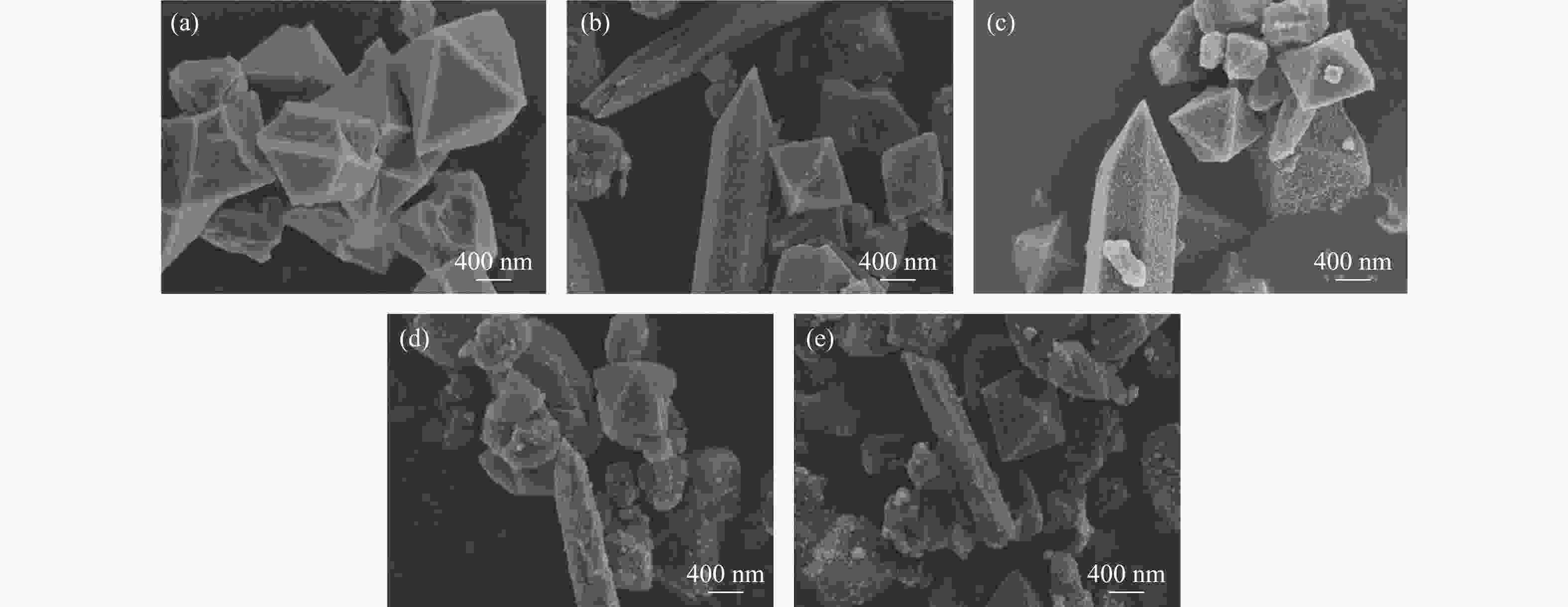
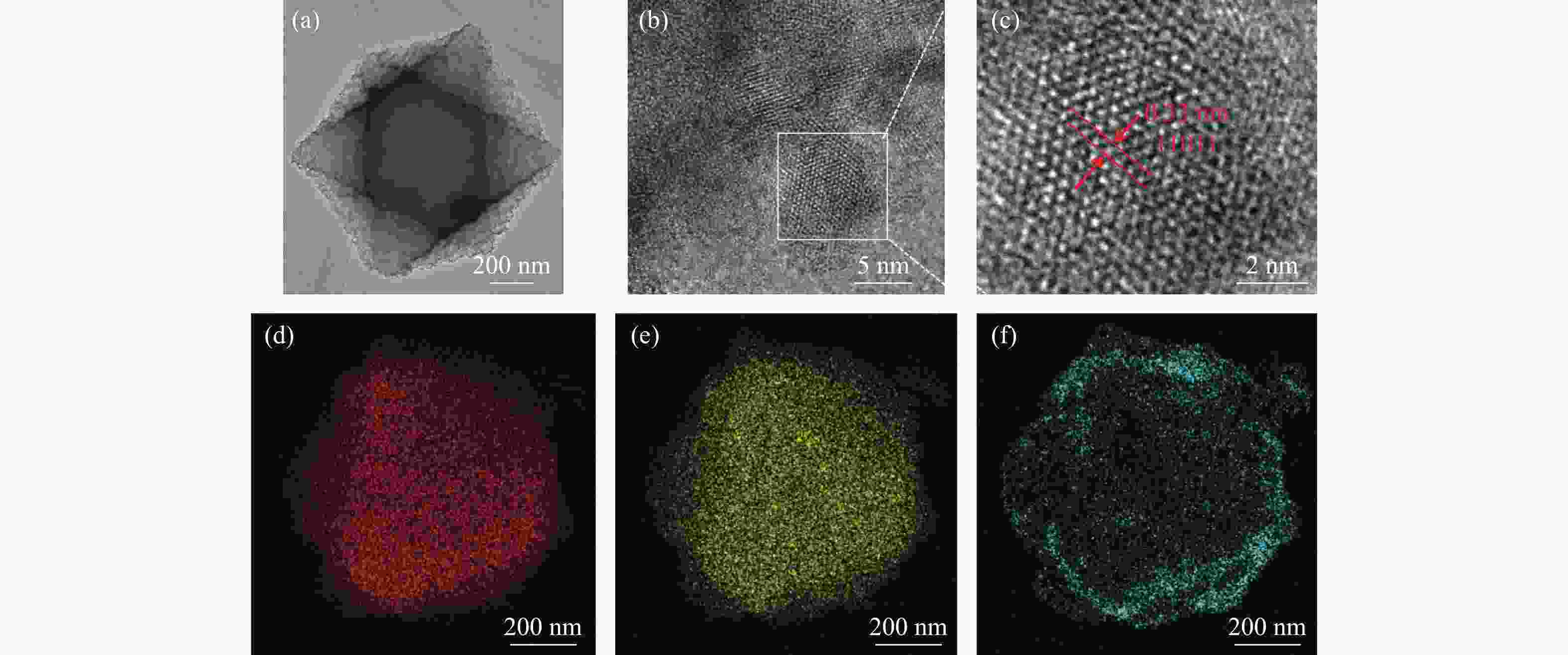
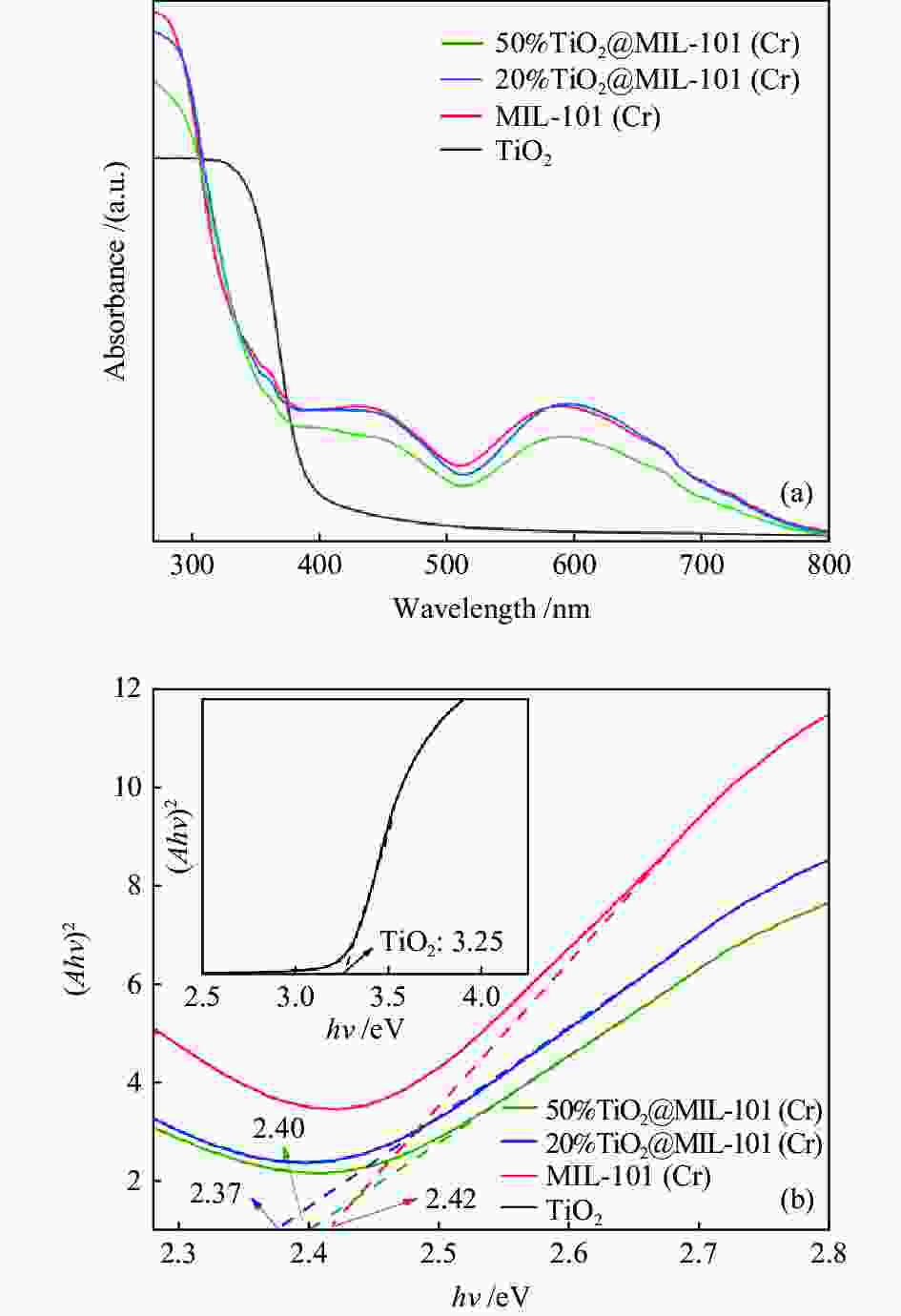
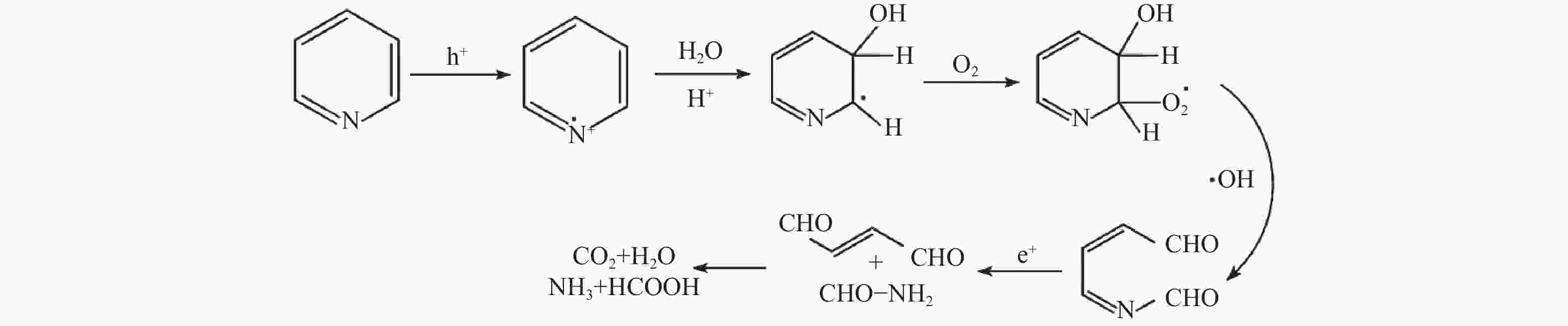
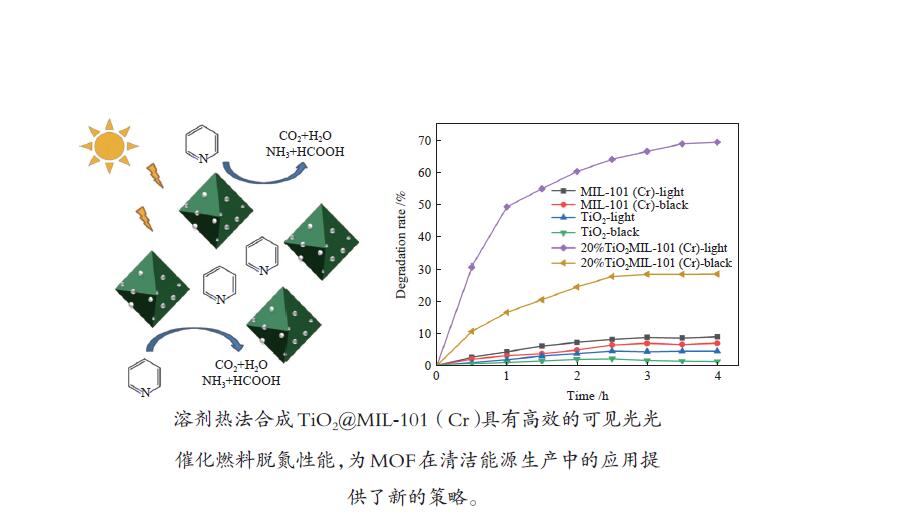
摘要: 溶剂热合成技术是一种有效合成复合材料的技术。在本研究中,通过溶剂热法制备了不同比例复合的TiO2@MIL-101(Cr)复合材料。并利用这些材料进行可见光光催化燃油脱氮(吡啶)。对所得催化剂采用XRD、FT-IR、SEM、TEM、BET和DRS进行表征。通过活性实验结果可以得到,20%TiO2@MIL-101(Cr)具有良好的催化活性,在可见光光照4 h后,对吡啶的脱除率高达70%。最后,通过对HPLC-MS数据分析,得到一个光催化燃油脱氮的可能机理。
-
关键词:
- 光催化 /
- 燃油 /
- 脱氮 /
- MIL-101(Cr) /
- TiO2
Abstract: Solvothermal synthesis technique is an effective method to create composite materials. In this paper, a series of TiO2@MIL-101(Cr) were prepared by the solvothermal method for photocatalytic denitrification of pyridine in fuel under visible light irradiation. The products were characterized by XRD, FT-IR, SEM, TEM, BET, DRS and ESR. The result shows that 20%TiO2@MIL-101(Cr) has high catalytic activity, the pyridine removal efficiency reaches values as high as 70% after irradiation for 240 min. Finally, we obtained the possible mechanism of photocatalytic denitrification according to the HPLC-MS spectrometry results analysis.-
Key words:
- photocatalytic /
- fuel /
- denitrification /
- MIL-101(Cr) /
- TiO2
-
Table 1 BET surface Area, pore volume of TiO2@MIL-101(Cr) composites
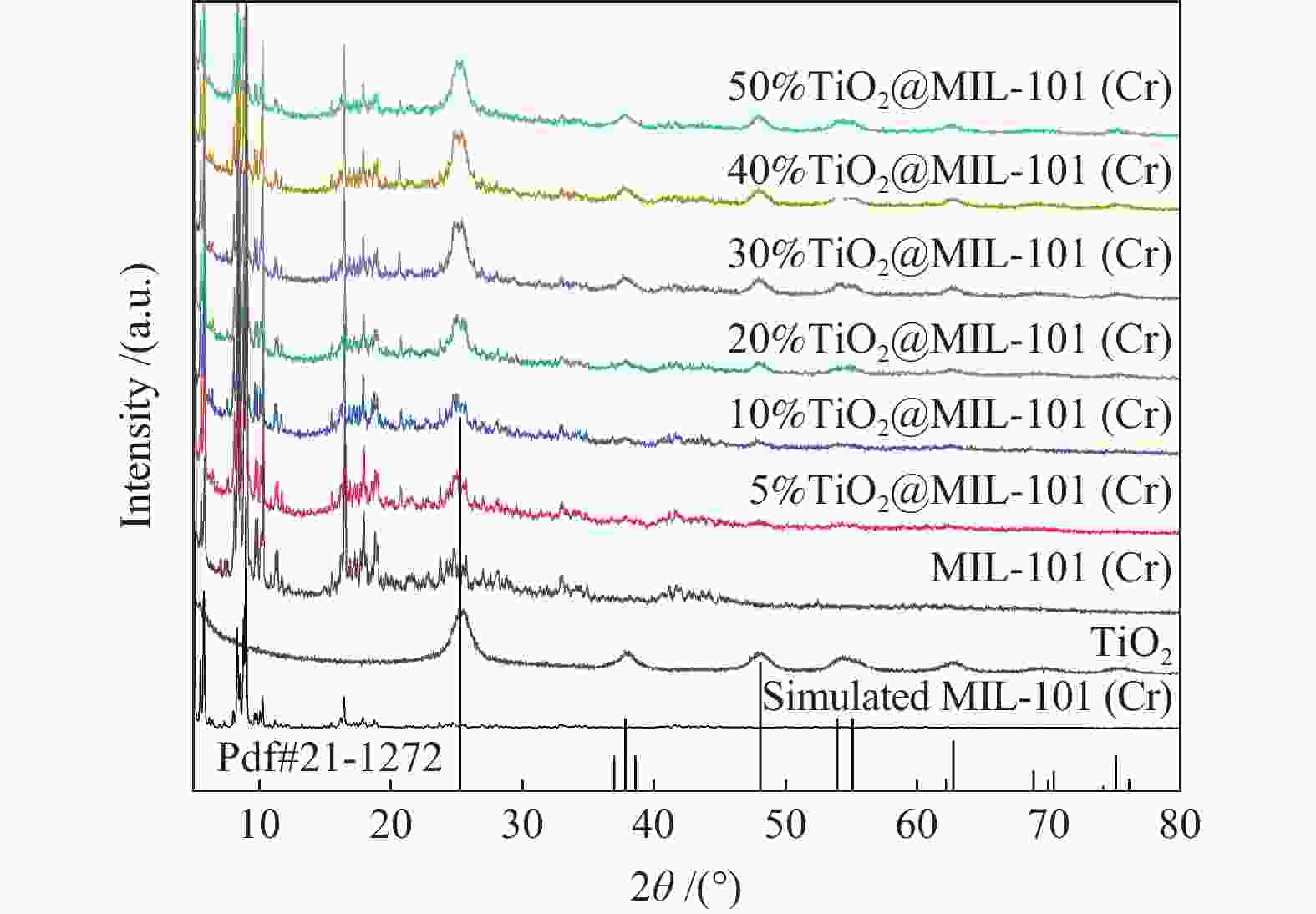
Sample BTE surface area/(m2·g−1) Pore volume/(cm3·g−1) MIL-101(Cr) 3341.1767 1.63 5%TiO2@MIL-101(Cr) 2855.3102 1.49 10%TiO2@MIL-101(Cr) 2585.1894 1.37 20%TiO2@MIL-101(Cr) 2325.2452 1.23 50%TiO2@MIL-101(Cr) 1742.2449 0.99 -
[1] YIN C. International law regulation of offshore oil and gas exploitation[J]. Environ Impact Assess Rev,2021,88:106551. [2] PRADO G H C, RAO Y, KLERK A. Nitrogen removal from oil: A review[J]. Energy Fuels,2016,31(1):14−36. [3] BHADRA B N, BAEK Y S, CHOI C H, JHUNG S H. How neutral nitrogen-containing compounds are oxidized in oxidative-denitrogenation of liquid fuel with TiO2@carbon[J]. Phys Chem Chem Phys,2021,23(14):8368−8374. doi: 10.1039/D1CP00633A [4] DEESE R D, MORRIS R E, METZ A E, MYERS K M, JOHNSON K, LOEGEL T N. Characterization of organic nitrogen compounds and their impact on the stability of marginally stable diesel fuels[J]. Energy Fuels,2019,33(7):6659−6669. doi: 10.1021/acs.energyfuels.9b00932 [5] PAUCAR N E, KIGGINS P, BLAD B, DE JESUS K, AFRIN F, PASHIKANTI S, SHARMA K. Ionic liquids for the removal of sulfur and nitrogen compounds in fuels: A review[J]. Environ Chem Lett,2021,19(2):1205−1228. doi: 10.1007/s10311-020-01135-1 [6] JURY M R. Meteorology of air pollution in Los Angeles[J]. Atmospheric Pollut Res,2020,11(7):1226−1237. doi: 10.1016/j.apr.2020.04.016 [7] LIANG R, HUANG R, WANG X, YING S, YAN G, WU L. Functionalized MIL-68(In) for the photocatalytic treatment of Cr(VI)-containing simulation wastewater: Electronic effects of ligand substitution[J]. Appl Surf Sci,2019,464:396−403. doi: 10.1016/j.apsusc.2018.09.100 [8] ALVARO M, CARBONELL E, FERRER B, LLABRES I XAMENA F X, GARCIA H. Semiconductor behavior of a metal-organic framework (MOF)[J]. Chem Eur J,2007,13(18):5106−51112. doi: 10.1002/chem.200601003 [9] DHAKSHINAMOORTHY A, LI Z, GARCIA H. Catalysis and photocatalysis by metal organic frameworks[J]. Chem Soc Rev,2018,47(22):8134−8172. doi: 10.1039/C8CS00256H [10] GAO P, LIU R, HUANG H, JIA X, PAN H. MOF-templated controllable synthesis of α-Fe2O3 porous nanorods and their gas sensing properties[J]. RSC Adv,2016,6(97):94699−94705. doi: 10.1039/C6RA21567J [11] LI Q, WU J, HUANG L, GAO J, ZHOU H, SHI Y, PAN Q, ZHANG G, DU Y, LIANG W. Sulfur dioxide gas-sensitive materials based on zeolitic imidazolate framework-derived carbon nanotubes[J]. J Mater Chem A,2018,6(25):12115−12124. doi: 10.1039/C8TA02036A [12] CUI Y F, JIANG W, LIANG S, ZHU L F, YAO Y W. MOF-derived synthesis of mesoporous In/Ga oxides and their ultra-sensitive ethanol-sensing properties[J]. J Mater Chem A,2018,6(30):14930−14938. doi: 10.1039/C8TA00269J [13] FANG Y, MA Y, ZHENG M, YANG P, ASIRI A M, WANG X. Metal-organic frameworks for solar energy conversion by photoredox catalysis[J]. Coord Chem Rev,2018,373:83−115. doi: 10.1016/j.ccr.2017.09.013 [14] YUAN S, FENG L, WANG K, PANG J, BOSCH M, LOLLAR C, SUN Y, QIN J, YANG X, ZHANG P, WANG Q, ZOU L, ZHANG Y, ZHANG L, FANG Y, LI J, ZHOU H C. Stable metal-organic frameworks: Design, synthesis, and applications[J]. Adv Mater,2018,30(37):e1704303. doi: 10.1002/adma.201704303 [15] XUE D-X, WANG Q, BAI J. Amide-functionalized metal-organic frameworks: Syntheses, structures and improved gas storage and separation properties[J]. Coord Chem Rev,2019,378:2−16. doi: 10.1016/j.ccr.2017.10.026 [16] YING M, TANG R, YANG W, LIANG W, YANG G, PAN H, LIAO X, HUANG J. Tailoring electronegativity of bimetallic Ni/Fe metal-organic framework nanosheets for electrocatalytic water oxidation[J]. ACS Appl Nano Mater,2021,4(2):1967−1975. doi: 10.1021/acsanm.0c03310 [17] LIANG R, HUANG R, YING S, WANG X, YAN G, WU L. Facile in situ growth of highly dispersed palladium on phosphotungstic-acid-encapsulated MIL-100(Fe) for the degradation of pharmaceuticals and personal care products under visible light[J]. J Nano Res,2017,11(2):1109−1123. [18] WANG C-C, DU X-D, LI J, GUO X-X, WANG P, ZHANG J. Photocatalytic Cr(VI) reduction in metal-organic frameworks: A mini-review[J]. App Catal B: Environ,2016,193:198−216. doi: 10.1016/j.apcatb.2016.04.030 [19] KHAN N A, JHUNG S H. Phytic acid-encapsulated MIL-101(Cr): Remarkable adsorbent for the removal of both neutral indole and basic quinoline from model liquid fuel[J]. Chem Eng J,2019,375. [20] GUO Q, ZHOU C, MA Z, YANG X. Fundamentals of TiO2 photocatalysis: Concepts, mechanisms, and challenges[J]. Adv Mater,2019,31(50):1901997. doi: 10.1002/adma.201901997 [21] FUJIMOTO T M, PONCZEK M, ROCHETTO U L, LANDERS R, TOMAZ E. Photocatalytic oxidation of selected gas-phase VOCs using UV light, TiO2, and TiO2/Pd[J]. Environ Sci Pollut Res Int,2017,24(7):6390−6396. doi: 10.1007/s11356-016-6494-7 [22] LIN J Y, LEE J, WEN D O, KWON E, LIN K. Hierarchical zif-decorated nanoflower-covered 3-dimensional foam for enhanced catalytic reduction of nitrogen-containing contaminants[J]. J Colloid Interface Sci 2021, 602: 95-104. [23] HU W, YAN G, LIANG R, JIANG M, HUANG R, XIA Y, CHEN L, LU Y. Construction of a novel step-scheme CdS/Pt/Bi2MoO6 photocatalyst for efficient photocatalytic fuel denitrification[J]. RSC Adv,2021,11(38):23288−23300. doi: 10.1039/D1RA04417F [24] LIANG R, LIANG I, CHEN F, XIE P, WV Y, WANG X, YAN G, WV L. Sodium dodecyl sulfate-decorated MOF-derived porous Fe2O3 nanoparticles: High performance, recyclable photocatalysts for fuel denitrification[J]. Chin J Catal,2020,41(1):188−199. [25] HUANG R, LIANG R, FAN H, YING S, WU L, WANG X, YAN G. Enhanced Photocatalytic fuel denitrification over TiO2/alpha-Fe2O3 nanocomposites under visible light irradiation[J]. Sci Rep,2017,7(1):7858. doi: 10.1038/s41598-017-08439-3 -




 下载:
下载: