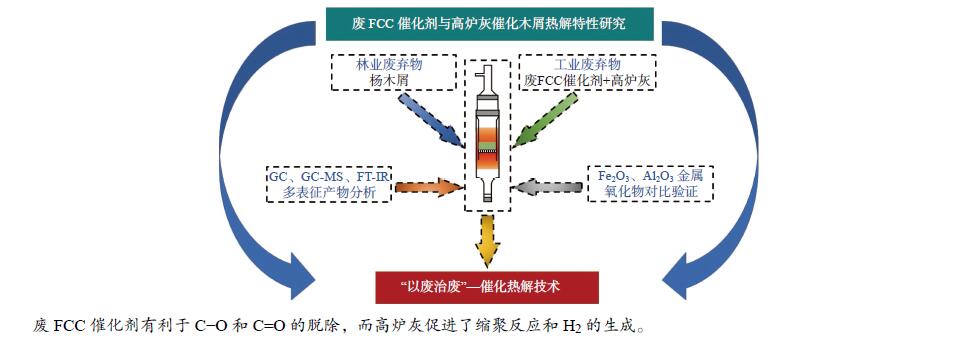
Study on the pyrolysis characteristics of sawdust catalyzed by spent FCC catalyst and blast furnace ash
-
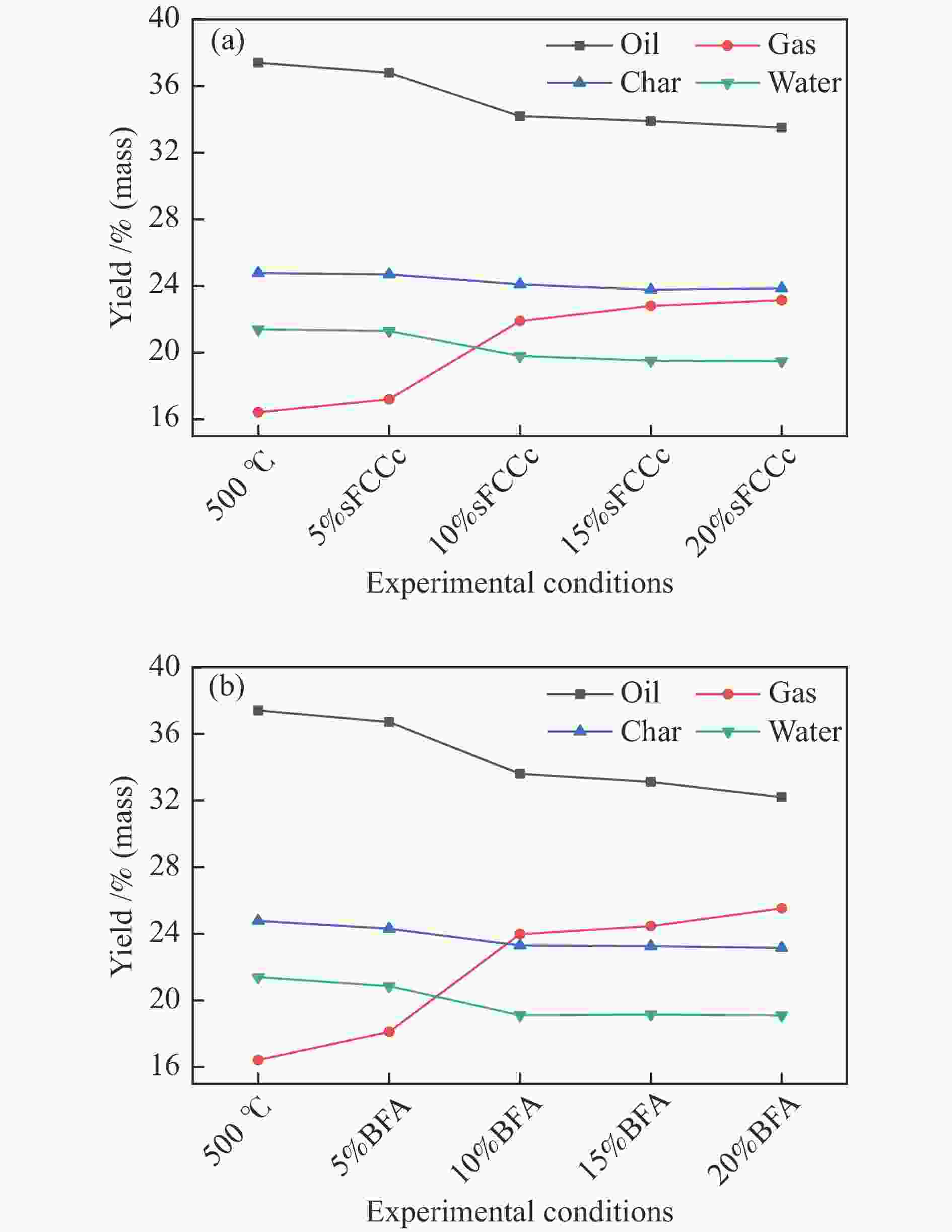
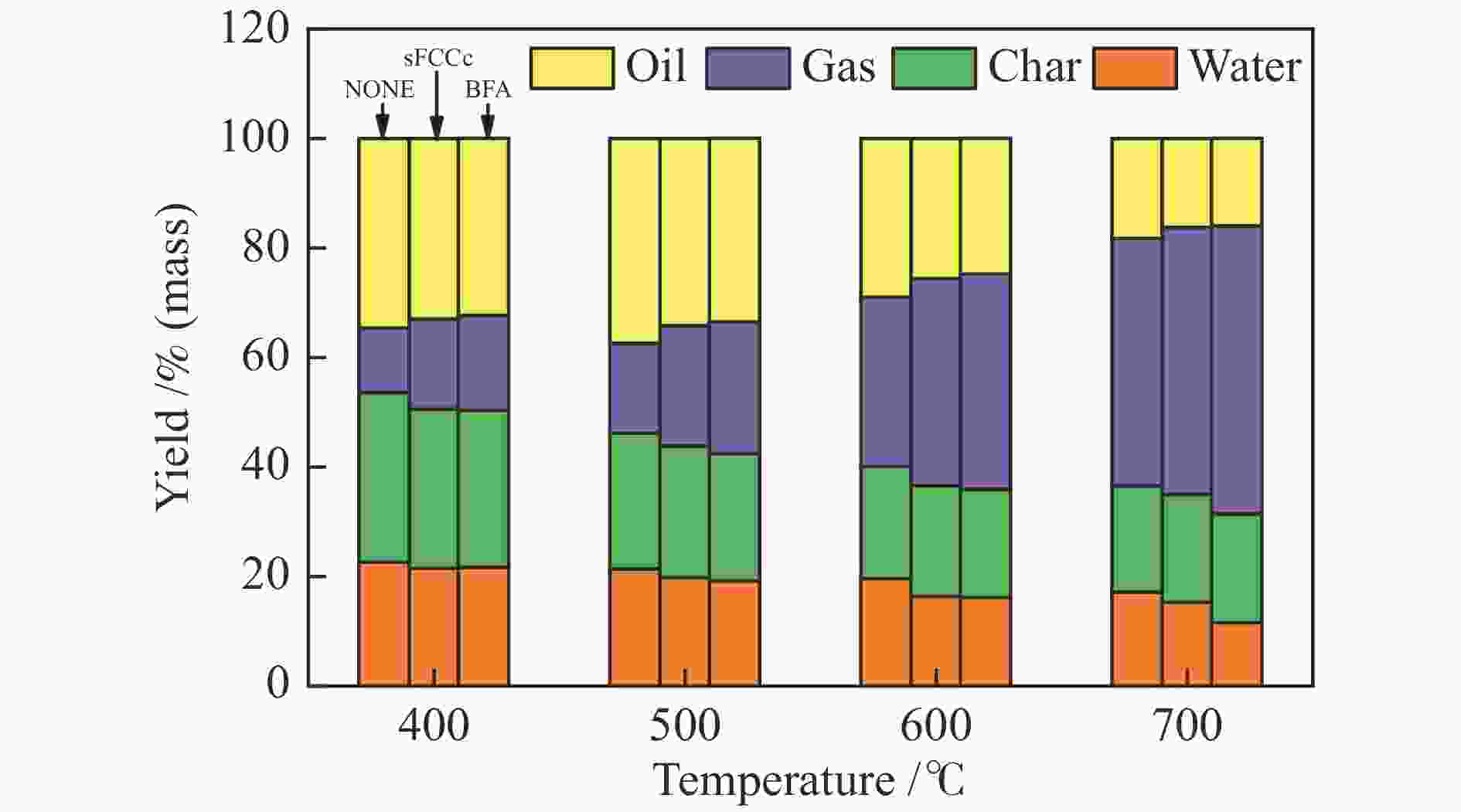
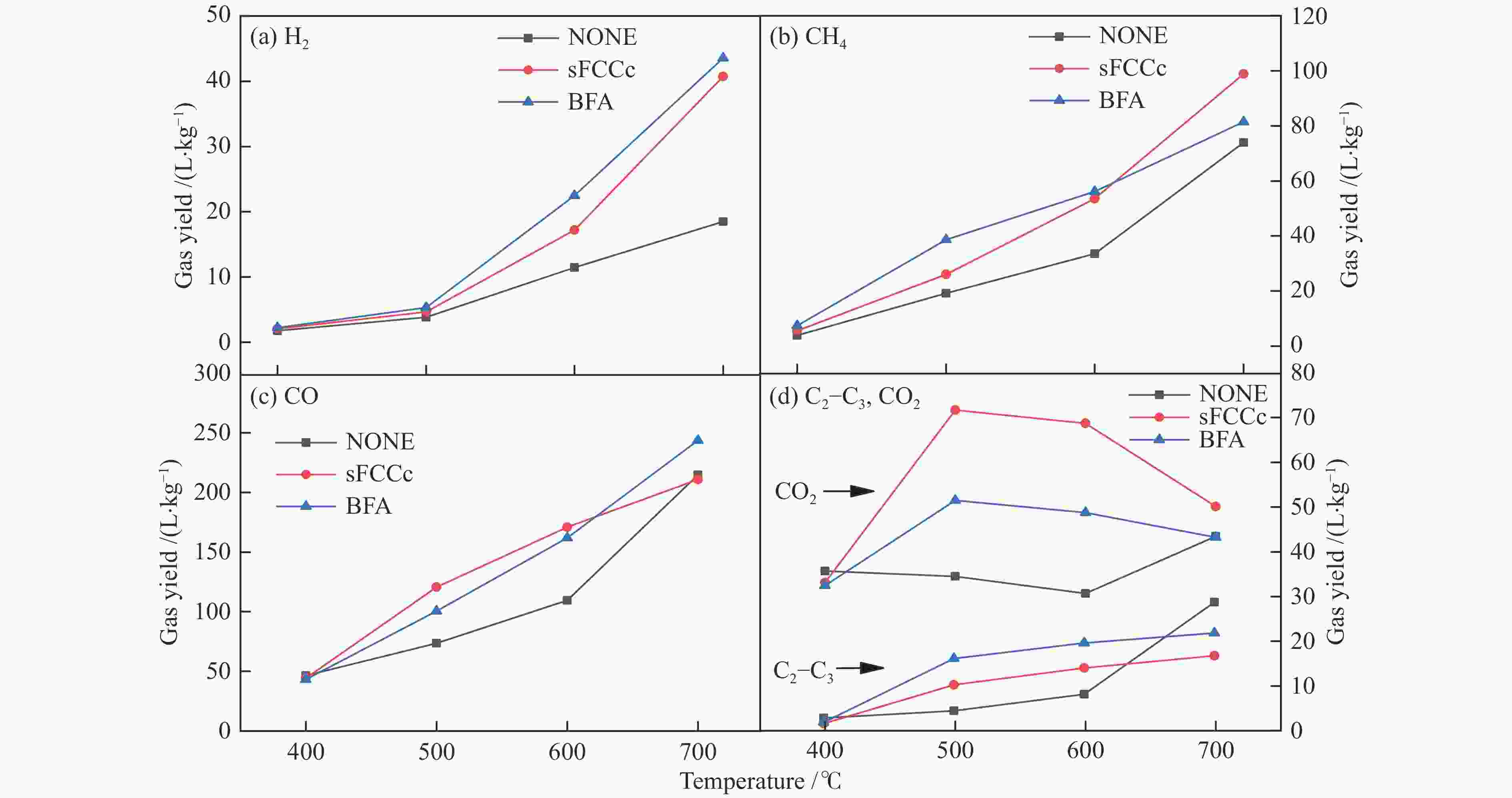
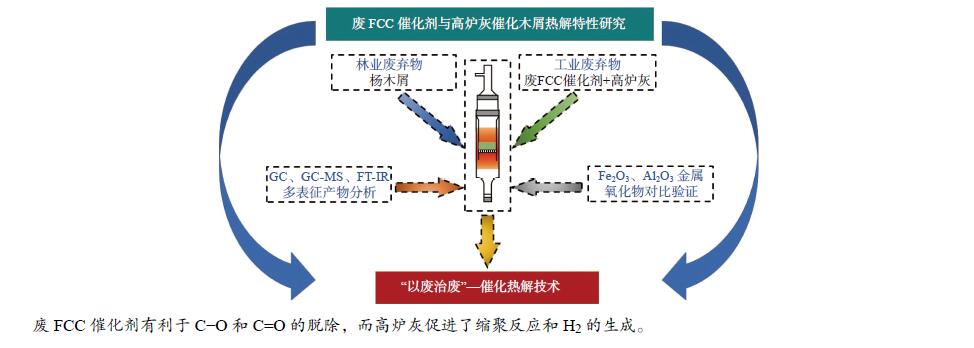
摘要: 将炼油废FCC催化剂(sFCCc)和炼钢高炉灰(BFA)两种典型工业废弃物作为催化剂应用于木屑快速热解过程中,探究了400−700 ℃木屑的催化热解反应特性。结果表明,两种催化剂均促进了液相产物向气相产物的转化,700 ℃、BFA催化条件下的气相产率最高为52.60%。sFCCc在500−600 ℃时具有更强的脱氧活性,气体产物中CO和CO2产量更高。BFA在600−700 ℃时具有更高的缩聚脱氢活性,催化生成了更大量的多环芳香类化合物和H2。热解油主要由酚类物质组成,sFCCc促进了甲氧基酚向苯二酚类物质转化。热解油FT-IR解析结果表明,sFCCc促进了C−O和C=O的脱除,导致酸类和酯类化合物减少,CO2产率增加。Abstract: Two industrial wastes, spent FCC catalyst (sFCCc) and blast furnace ash (BFA), were used as catalysts in the fast pyrolysis of sawdust, and the catalytic pyrolysis reaction characteristics of sawdust in the temperature range of 400−700 ℃ were explored. The results showed that both catalysts promoted the conversion from liquid products to gaseous products, and the highest gas yield was 52.60% at 700 ℃ catalyzed by BFA. The sFCCc had stronger deoxygenation activity at 500−600 ℃, resulting in higher CO and CO2 production in gaseous products. While BFA had higher polycondensation and dehydrogenation activity at 600−700 ℃, and promoted the formation of polycyclic aromatic compounds and H2. Pyrolysis oil was mainly composed of phenols. The sFCCc promoted the conversion of methoxy phenol to benzenediol. FT-IR analysis of pyrolysis oil showed that sFCCc promoted the removal of C−O and C=O, resulting in decreased acid and ester compounds and increased CO2 yield.
-
Key words:
- biomass /
- pyrolysis /
- spent FCC catalyst /
- blast furnace ash
-
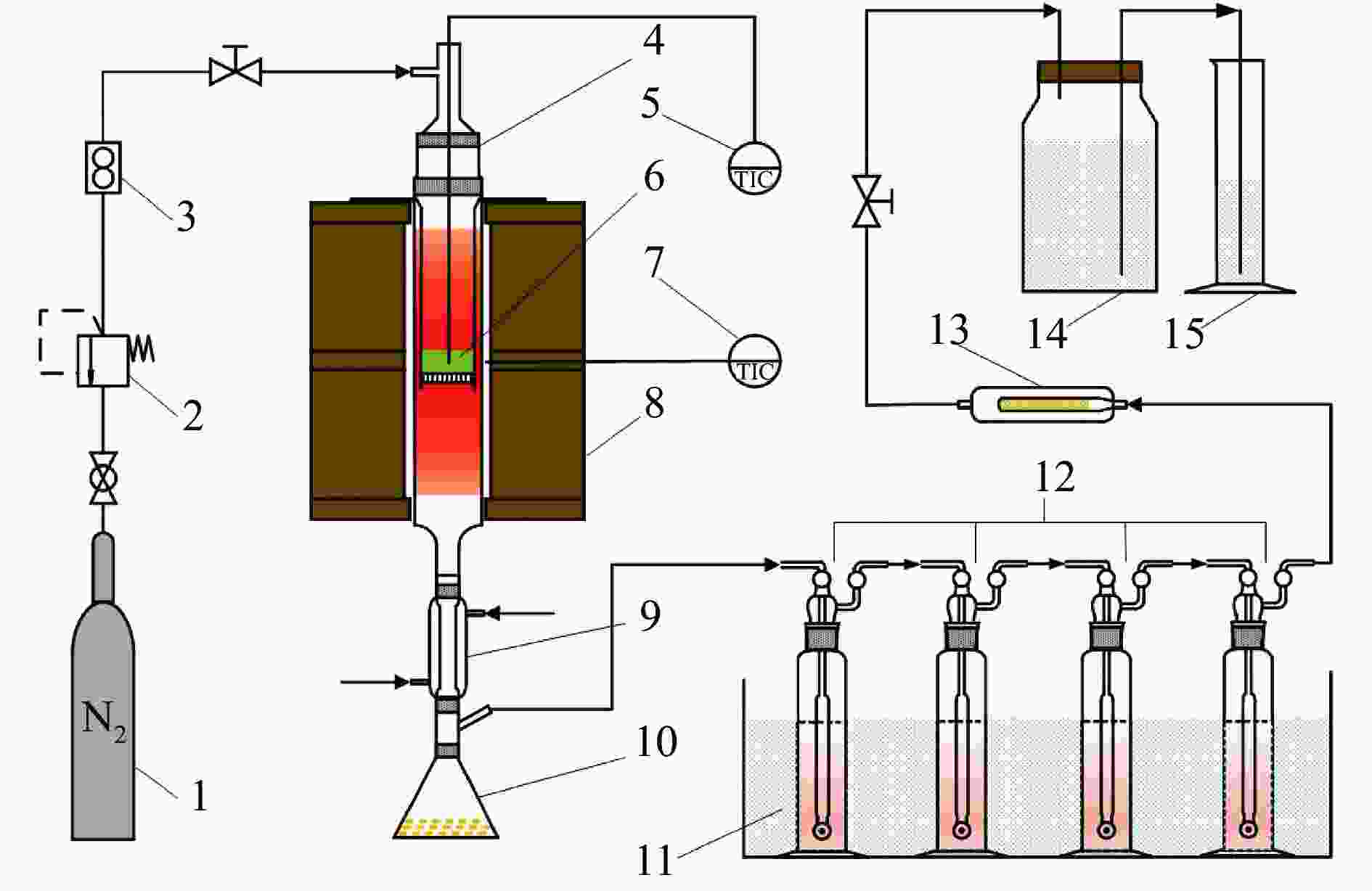
图 1 实验装置流程示意图
Figure 1 Schematic diagram of experimental apparatus
1: Nitrogen cylinder; 2: Reducing valve; 3: Mass flowmeter; 4: Quartz reactor; 5: Thermocouple; 6: Biomass; 7: Temperature control thermocouple; 8: Furnace; 9: Condenser tube; 10: Erlenmeyer flask; 11: Ice water bath; 12: Acetone bottle washing set; 13: Sleeve filter; 14: Gas cylinder; 15: Graduated cylinder
表 1 杨木屑的工业分析和元素分析
Table 1 Proximate and ultimate analyses of the poplar sawdust
Proximate analysis wad/% Ultimate analysis wdaf/% A V FC M C H S N O* 1.50 77.71 17.90 2.89 46.80 6.73 0.14 0.23 46.10 ad: air-dried basis; daf: dried ash-free basis; *: calculated by difference 表 2 BFA和sFCCc所含主要金属氧化物
Table 2 Main metal oxides in BFA and SFCCc
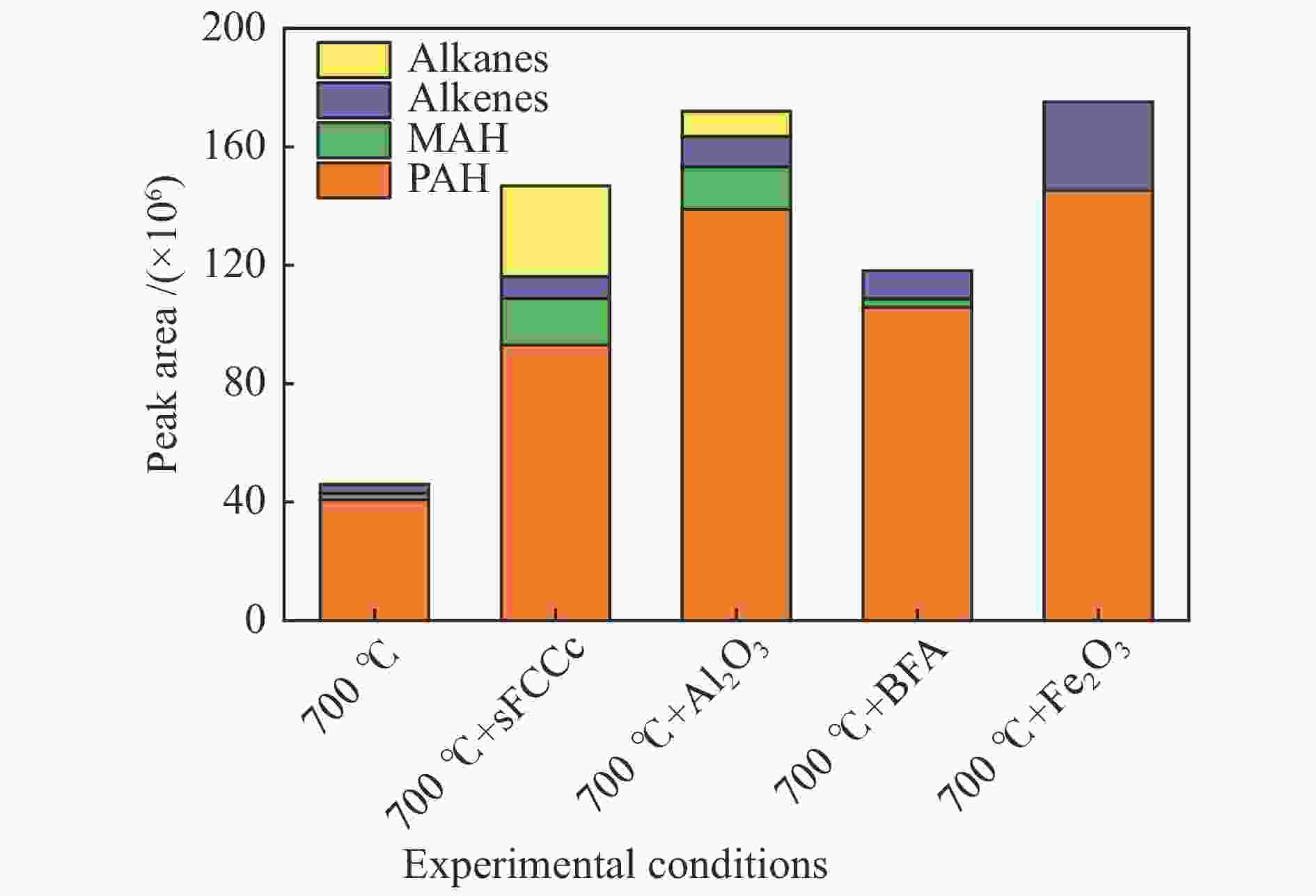
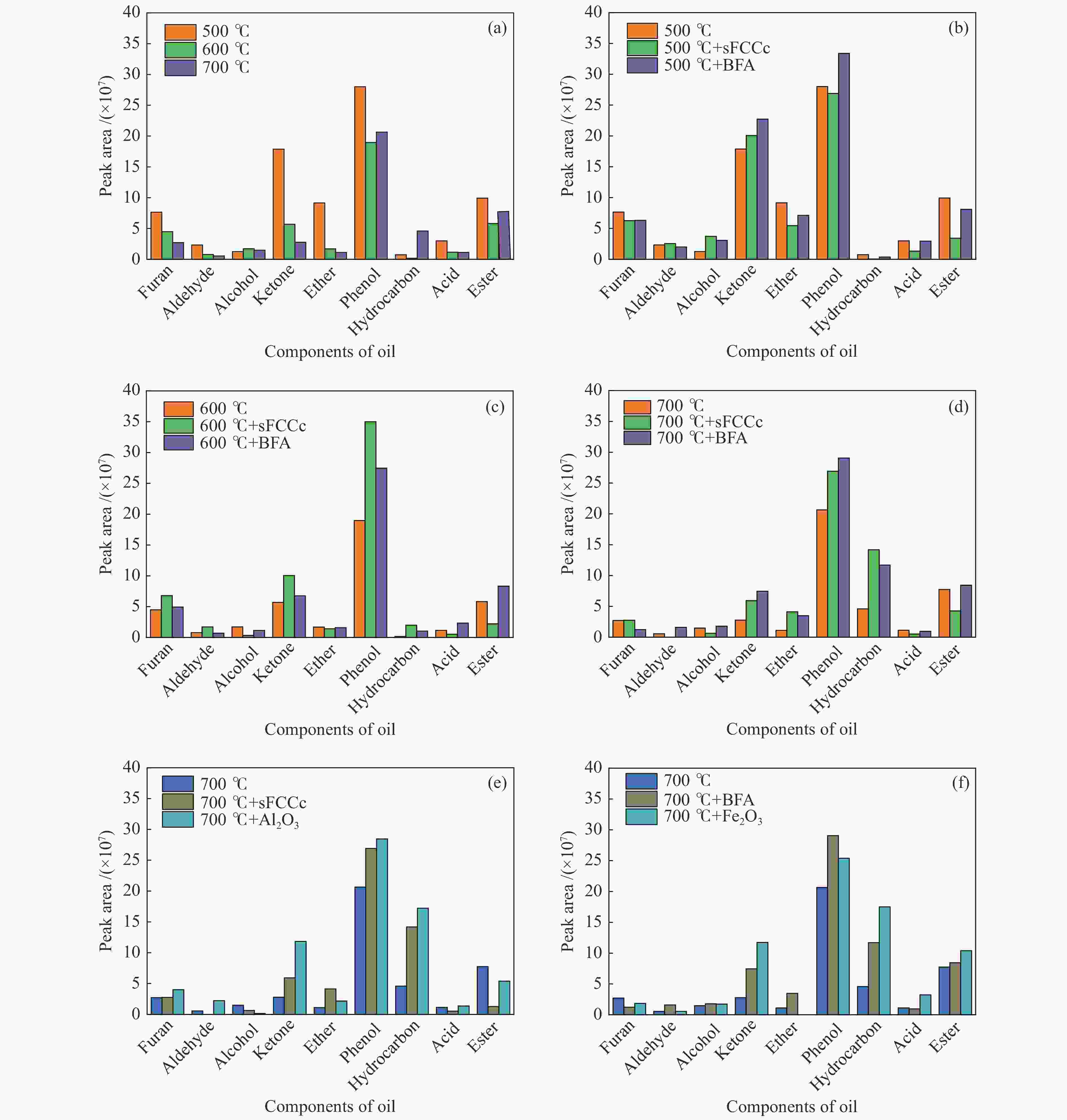
Industrial waste Fe2O3 Al2O3 SiO2 K2O CaO Na2O MgO BFA/% (mass) 60.60 3.85 9.73 4.74 7.67 3.33 2.02 sFCCc/% (mass) 0.56 45.56 44.20 0.15 0.16 0.26 0.16 表 3 不同温度和催化条件下主要酚类物质绝对峰面积
Table 3 Absolute peak area of main phenols under different temperature and catalytic conditions
Name Experimental condition /Absolute peak area (×106) 500 ℃ 500 ℃
sFCCc500 ℃
BFA600 ℃ 600 ℃
sFCCc600 ℃
BFA700 ℃ 700 ℃
sFCCc700 ℃
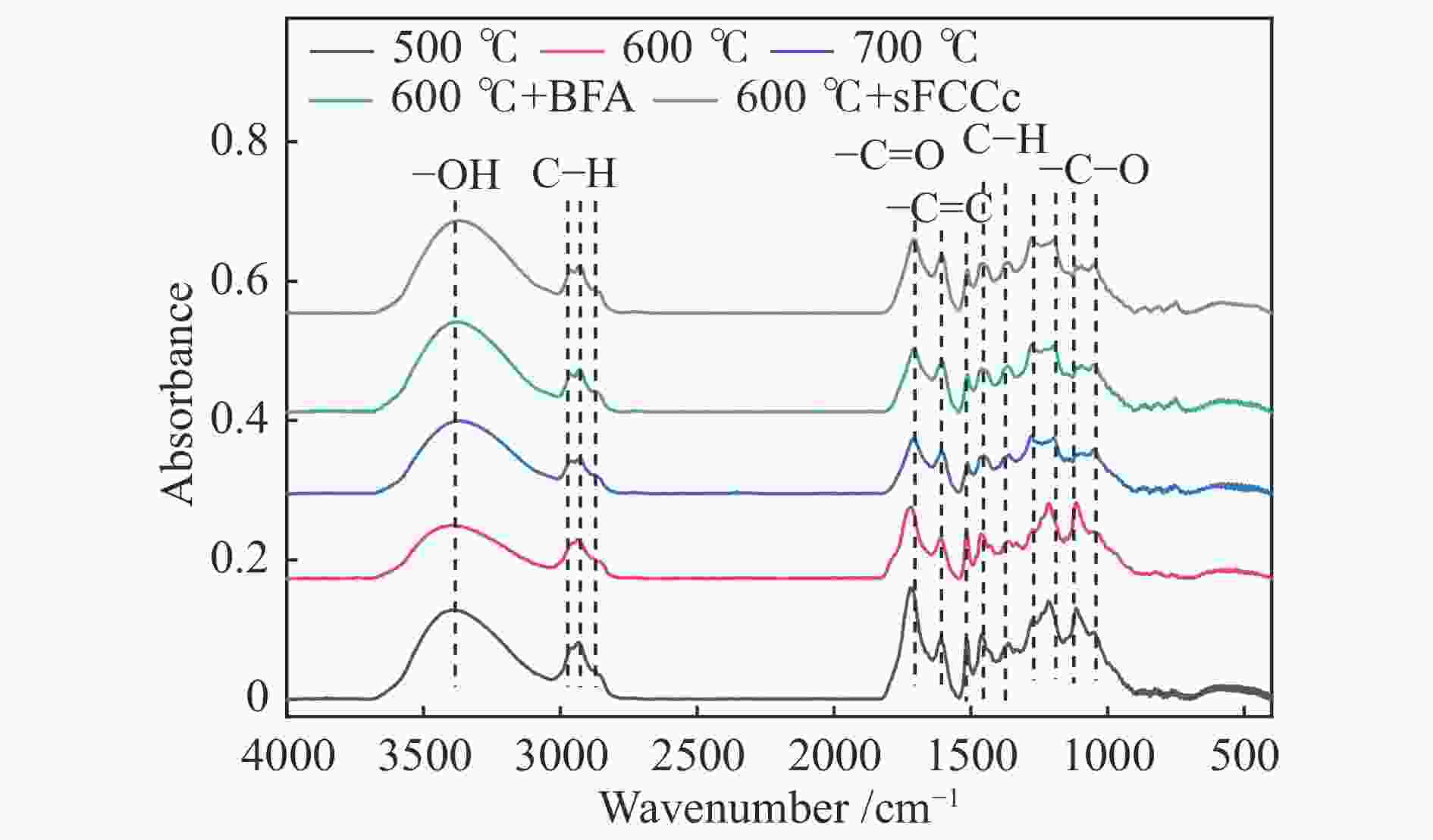
BFAPhenol 5.85 4.31 6.17 6.39 13.85 15.17 19.46 52.08 57.10 Phenol-methyl, 2- 8.75 5.76 6.64 8.48 15.12 14.93 19.02 37.40 31.77 Phenol, 3-methyl- Na Na Na 11.16 25.79 19.38 27.16 32.53 48.02 Phenol, 2,3-dimethyl- 4.11 3.89 Na 3.77 34.01 15.89 6.15 7.58 16.67 Phenol, 3,5-dimethyl- 5.44 8.78 Na 11.02 6.91 7.78 16.28 26.38 20.55 Phenol, 2-methoxy-4-methyl- 17.86 7.78 25.81 Na Na Na Na Na Na Phenol, 2,6-dimethoxy- 51.95 37.31 52.34 Na Na Na Na Na Na Phenol, 2,6-dimethoxy-4-(2-propenyl)- 40.20 11.70 44.94 Na Na Na Na Na Na Phenol, 2-methoxy- 75.78 30.49 37.77 Na Na Na Na Na Na 1,2-benzenediol 15.36 50.24 46.36 42.64 54.83 56.85 44.30 17.03 35.85 1,2-benzenediol, 4-methyl- Na Na Na 59.81 21.14 20.41 8.61 Na 12.60 *Na: not available Peak positions
/cm−1Half peak
width/cm−1Functional
groupsPeak area 500 ℃ 600 ℃ 700 ℃ 600 ℃-
sFCCc600 ℃-
BFA3444
3286106
167O−H stretching
vibration band48.27 28.87 39.85 48.41 50.00 2939
2854
1461
144650
23
9
44C−H stretching
and bending
vibration peaks21.02 13.59 8.73 10.41 11.40 1773
171827
30C=O vibration
peaks12.04 8.41 5.61 4.93 7.31 1660
1607
151423
25
9C=C stretching
vibration peak in
aromatic rings7.97 5.00 5.58 7.28 7.65 1363
1325
1283
1214
1154
1113
105427
15
27
43
13
27
34C−O stretching
vibration peak34.34 25.01 21.60 23.65 30.43 -
[1] 国家林业和草原局. 中国森林资源报告(2014-2018)[M]. 北京: 中国林业出版社, 2019.National Forestry and Grassland Administration. China Forest Resources Report (2014-2018)[M]. Beijing: China Forestry Press, 2019. [2] UZOEJINWA B B, HE X, WANG S, ABOMOHRA A E, HU Y M, WANG Q. Co-pyrolysis of biomass and waste plastics as a thermochemical conversion technology for high-grade biofuel production: Recent progress and future directions elsewhere worldwide[J]. Energy Convers Manage,2018,163:468−492. doi: 10.1016/j.enconman.2018.02.004 [3] 李承宇, 张军, 袁浩然, 王树荣, 陈勇. 纤维素热解转化的研究进展[J]. 燃料化学学报,2021,49(12):1733−1751. doi: 10.1016/S1872-5813(21)60134-2LI Cheng-yu, ZHANG Jun, YUAN Hao-ran, WANG Shu-rong, CHEN Yong. Research progress of cellulose pyrolysis conversion[J]. J Fuel Chem Technol,2021,49(12):1733−1751. doi: 10.1016/S1872-5813(21)60134-2 [4] ARNI S A. Comparison of slow and fast pyrolysis for converting biomass into fuel[J]. Renewable Energ,2018,124:197−201. doi: 10.1016/j.renene.2017.04.060 [5] HU X, GUNAWAN R, MOURANT D, LIEVENS C, LI X, ZHANG S, CHAIWAT W, LI C Z. Acid-catalysed reactions between methanol and the bio-oil from the fast pyrolysis of mallee bark[J]. Fuel,2012,97:512−522. doi: 10.1016/j.fuel.2012.02.032 [6] ZHANG X D, SUN L Z, CHEN L, XIE X P, ZHAO B F, SI H Y, MENG G F. Comparison of catalytic upgrading of biomass fast pyrolysis vapors over CaO and Fe (III)/CaO catalysts[J]. J Anal Appl Pyrolysis,2014,108:35−40. doi: 10.1016/j.jaap.2014.05.020 [7] DAI L L, WANG Y P, LIU Y H, ROGER R, DUAN D L, ZHAO Y F, YU Z T, JIANG L. Catalytic fast pyrolysis of torrefied corn cob to aromatic hydrocarbons over Ni-modified hierarchical ZSM-5 catalyst[J]. Bioresour Technol,2019,272:407−414. doi: 10.1016/j.biortech.2018.10.062 [8] 方书起, 石崇, 李攀, 白净, 常春. Fe-Zn共改性ZSM-5催化作用下生物质快速热解特性研究[J]. 化工学报,2020,71(4):1637−1645.FANG Shu-qi, SHI Chong, LI Pan, BAI Jing, CHANG Chun. Study on the characteristics of fast pyrolysis of biomass under the catalysis of Fe-Zn co-modified ZSM-5[J]. J Chem Ind Eng,2020,71(4):1637−1645. [9] PAYSEPAR H, RAO K T V, YUAN Z Y, YUAN Z S, SHUI H F, XU C B. Improving activity of ZSM-5 zeolite catalyst for the production of monomeric aromatics/phenolics from hydrolysis lignin via catalytic fast pyrolysis[J]. Appl Catal A: Gen,2018,563:154−162. doi: 10.1016/j.apcata.2018.07.003 [10] LIU Q, WANG J Z, ZHOU J, YU Z W. Promotion of monocyclic aromatics by catalytic fast pyrolysis of biomass with modified HZSM-5[J]. J Anal Appl Pyrolysis,2021,153:104964. doi: 10.1016/j.jaap.2020.104964 [11] CHE Q F, YANG M J, WANG X H, YANG Q, WILLIAMS L R, YANG H P, ZOU J, ZENG K, ZHU Y J, CHEN Y Q, CHEN H P. Influence of physicochemical properties of metal modified ZSM-5 catalyst on benzene, toluene and xylene production from biomass catalytic pyrolysis[J]. Bioresour Technol,2019,278:248−254. doi: 10.1016/j.biortech.2019.01.081 [12] YANG M F, SHAO J G, YANG Z X, YANG H P, WANG X H, WU Z S, CHEN H P. Conversion of lignin into light olefins and aromatics over Fe/ZSM-5 catalytic fast pyrolysis: Significance of Fe contents and temperature[J]. J Anal Appl Pyrolysis,2019,137:259−265. doi: 10.1016/j.jaap.2018.12.003 [13] 马会霞, 周峰, 武光, 傅杰, 乔凯. 多级孔HZSM-5分子筛催化快速热解生物质制芳烃[J]. 化工学报,2020,71(11):5200−5207.MA Hui-xia, ZHOU Feng, WU Guang, FU Jie, QIAO Kai. Hierarchical pore HZSM-5 molecular sieve catalyzed rapid pyrolysis of biomass to aromatics[J]. J Chem Ind Eng,2020,71(11):5200−5207. [14] 王在花, 李琰, 马艳萍. 炼油废催化剂的回收利用现状研究[J]. 化工管理,2019,(34):166−167. doi: 10.3969/j.issn.1008-4800.2019.34.090WANG Zai-hua, LI Yan, MA Yan-ping. Research on the current situation of recycling and utilization of waste catalysts in oil refining[J]. Chem Enterp Manage,2019,(34):166−167. doi: 10.3969/j.issn.1008-4800.2019.34.090 [15] RO D, KIM Y M, LEE I G, JAE J, JUNG S C, KIM S C, PARK Y K. Bench scale catalytic fast pyrolysis of empty fruit bunches over low cost catalysts and HZSM-5 using a fixed bed reactor[J]. J Clean Prod,2018,176:298−303. doi: 10.1016/j.jclepro.2017.12.075 [16] WANG Q H, LI Y, CHELSEA B, LI Y M, CHEN C M, AN Z X, MOHAMED G E D. Spent fluid catalytic cracking (FCC) catalyst enhances pyrolysis of refinery waste activated sludge[J]. J Clean Prod,2021,295:126382. doi: 10.1016/j.jclepro.2021.126382 [17] HUANG Z H, QIN L B, XU Z, CHEN W S, XING F T, HAN J. The effects of Fe2O3 catalyst on the conversion of organic matter and bio-fuel production during pyrolysis of sewage sludge[J]. J Energy Inst,2019,92:835−842. doi: 10.1016/j.joei.2018.06.015 [18] SONG Q, ZHAO H Y, MA Q X, YANG L, MA L, WU Y, ZHANG P. Catalytic upgrading of coal volatiles with Fe2O3 and hematite by TG-FTIR and Py-GC/MS[J]. Fuel,2021,92(4):835−842. [19] LIN Y Y, ZHANG C, ZHANG M C, ZHANG J. Deoxygenation of bio-oil during pyrolysis of biomass in the presence of CaO in a fluidized-bed reactor[J]. Energy Fuels,2010,24(10):5686−5695. doi: 10.1021/ef1009605 [20] YUAN R, SHEN Y F. Catalytic pyrolysis of biomass-plastic wastes in the presence of MgO and MgCO3 for hydrocarbon-rich oils production[J]. Bioresour Technol,2019,293:122076. doi: 10.1016/j.biortech.2019.122076 [21] 崔石岩, 张明慧, 孙永峰, 蒋曼, 高恩霞, 卢中博. 高炉灰与赤泥共还原—磁选回收铁试验研究[J]. 金属矿山,2020,(3):102−107. doi: 10.19614/j.cnki.jsks.202003015CUI Shi-yan, ZHANG Ming-hui, SUN Yong-feng, JIANG Man, GAO En-xia, LU Zhong-bo. Experimental study on co-reduction of blast furnace ash and red mud - magnetic separation for iron recovery[J]. Met Min,2020,(3):102−107. doi: 10.19614/j.cnki.jsks.202003015 [22] PANG Y J, WU D, CHEN Y S, XU J, WU J R, ZHAI M S. Pyrolysis of pine pellets catalyzed by blast furnace gas ash[J]. Chem Eng Process,2020,156:108094. doi: 10.1016/j.cep.2020.108094 [23] 刘志超, 仲兆平, 丁宽, 张波. 松木屑催化热解及热解油分析[J]. 燃烧科学与技术,2014,20(1):91−94.LIU Zhi-chao, ZHONG Zhao-ping, DING Kuan, ZHANG Bo. Catalytic pyrolysis of pine sawdust and analysis of pyrolysis oil[J]. J Combust Sci Technol,2014,20(1):91−94. [24] 黄金保. 纤维素快速热解机理的分子模拟研究[D]. 重庆: 重庆大学, 2010.HUANG Jin-bao. Molecular simulation study on the rapid pyrolysis mechanism of cellulose[D]. Chongqing: Chongqing University, 2010. [25] HU C S, LIU C, LIU Q Y, ZHANG H Y, WU S L, XIAO R. Effects of steam to enhance the production of light olefins from ex-situ catalytic fast pyrolysis of biomass[J]. Fuel Process Technol,2020,210:106562. doi: 10.1016/j.fuproc.2020.106562 [26] ADENIYI A G, OTOIKHIAN K S, IGHALO J O. Steam reforming of biomass pyrolysis oil: A review[J]. Int J Chem React Eng,2019,17(4):20180328. [27] FRENCH R, CZERNIK S. Catalytic pyrolysis of biomass for biofuels production[J]. Fuel Process Technol,2010,91(1):25−32. doi: 10.1016/j.fuproc.2009.08.011 [28] ARENILLAS A, RUBIERA F, PIS J J, CUESTA M J, LGLESIAS M J, JIMENEZ A, SUAREZ-RUIZ I. Thermal behaviour during the pyrolysis of low rank perhydrous coals[J]. J Anal Appl Pyrolysis,2003,68(03):371−385. [29] YANG J K, XU X Y, SHA L, GUAN R N, LI H S, CHEN Y, LIU B C, SONG J, YU W B, XIAO K K, HOU H J, HU J P, YAO H, XIAO B. Enhanced hydrogen production in catalytic pyrolysis of sewage sludge by red mud: Thermogravimetric kinetic analysis and pyrolysis characteristics[J]. Int J Hydrogen Energy, 43(16): 7795−7807. [30] LOY A C M, YUSUP S, LAM M K, CHIN B L F, SHAHBAJ M, YAMAMOTO A, ACDA M N. The effect of industrial waste coal bottom ash as catalyst in catalytic pyrolysis of rice husk for syngas production[J]. Energy Convers Manage,2018,165:541−554. doi: 10.1016/j.enconman.2018.03.063 [31] FU P, YI W M, BAI X Y, LI Z H, HU S, XIANG J. Effect of temperature on gas composition and char structural features of pyrolyzed agricultural residues[J]. Bioresour Technol,2011,102(17):8211−8219. doi: 10.1016/j.biortech.2011.05.083 [32] ATIENZA M M, RUBIO I, FONTS I, CEAMANOS J, GEA G. Effect of torrefaction on the catalytic post-treatment of sewage sludge pyrolysis vaporsusing γ-Al2O3[J]. Chem Eng J,2017,308:264−274. doi: 10.1016/j.cej.2016.09.042 [33] 戴贡鑫, 王冠宇, 王凯歌, 朱玲君, 王树荣. 2, 6-二甲氧基苯酚热解机理研究[J]. 燃烧科学与技术,2020,26(6):501−506.DAI Gong-xing, WANG Guan-yu, WANG Kai-ge, ZHU Ling-jun, WANG Shu-rong. Study on the pyrolysis mechanism of 2, 6-dimethoxyphenol[J]. J Combust Sci Technol,2020,26(6):501−506. [34] AMEN-CHEN C, PAKDEL H, ROY C. Production of monomeric phenols by thermochemical conversion of biomass: a review[J]. Bioresour Technol,2001,79(3):277−299. doi: 10.1016/S0960-8524(00)00180-2 [35] FERRARI M, BOSMANS S, MAGGI R, DELMON B, GRANGR P. CoMo/carbon hydrodeoxygenation catalysts: influence of the hydrogen sulfide partial pressure and of the sulfidation temperature[J]. Catal Today,2001,65(2):257−264. [36] WU D, LIU G J, SUN R Y, XIANG F. Investigation of structural characteristics of thermally metamorphosed coal by FTIR spectroscopy and x-ray diffraction[J]. Energy Fuels,2013,27(10):5823−5830. doi: 10.1021/ef401276h [37] LIEVENS C, MOURANT D, HE M, GUNAWAN R, LI X, LI C Z. An FT-IR spectroscopic study of carbonyl functionalities in bio-oils[J]. Fuel, 90(11): 3417-3423. [38] PAINTER P C, SNYDER R W, STARSINIC M, COLEMAN M M, KUEHN D W, DAVIS A. Concerning the application of FT-IR to the study of coal: a critical assessment of band assign-ments and the application of spectral analysis programs[J]. Appl Spectrosc,1981,35(5):475−485. doi: 10.1366/0003702814732256 [39] JIANG J Y, YANG W H, CHENG Y P, LIU Z D, ZHANG Q, ZHAO K. Molecular structure characterization of middle-high rank coal via XRD, Raman and FTIR spectroscopy: Implications for coalification[J]. Fuel,2019,239:559−572. doi: 10.1016/j.fuel.2018.11.057 -




 下载:
下载: