Deactivation mechanism of Cu/SiO2 catalyst in gas phase hydrogenation of furfural to furfuryl alcohol
-
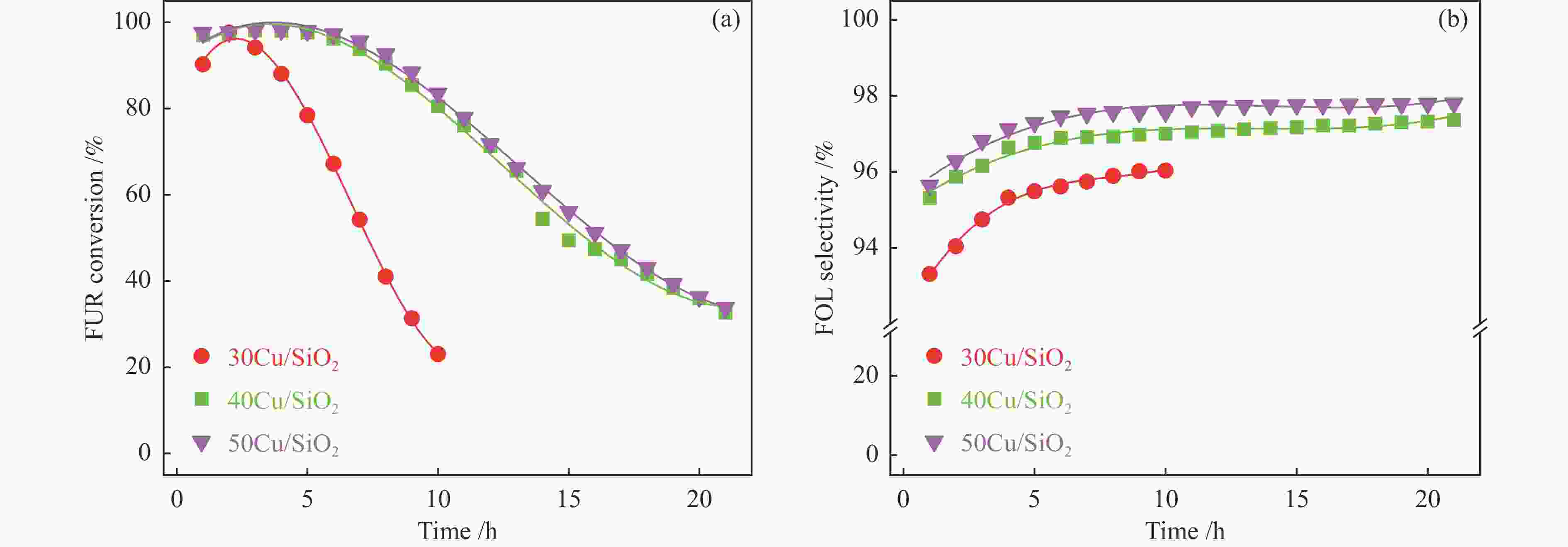
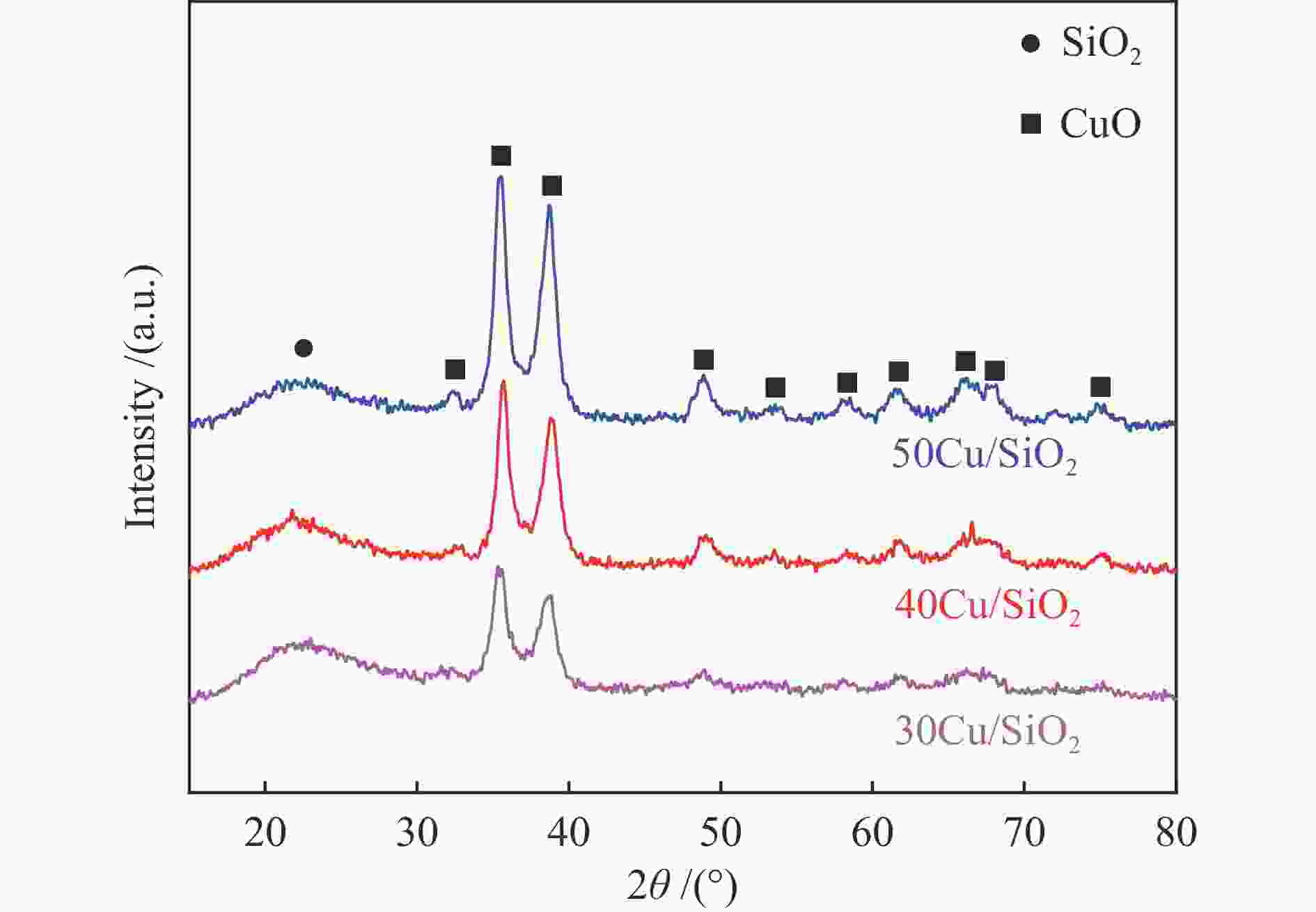
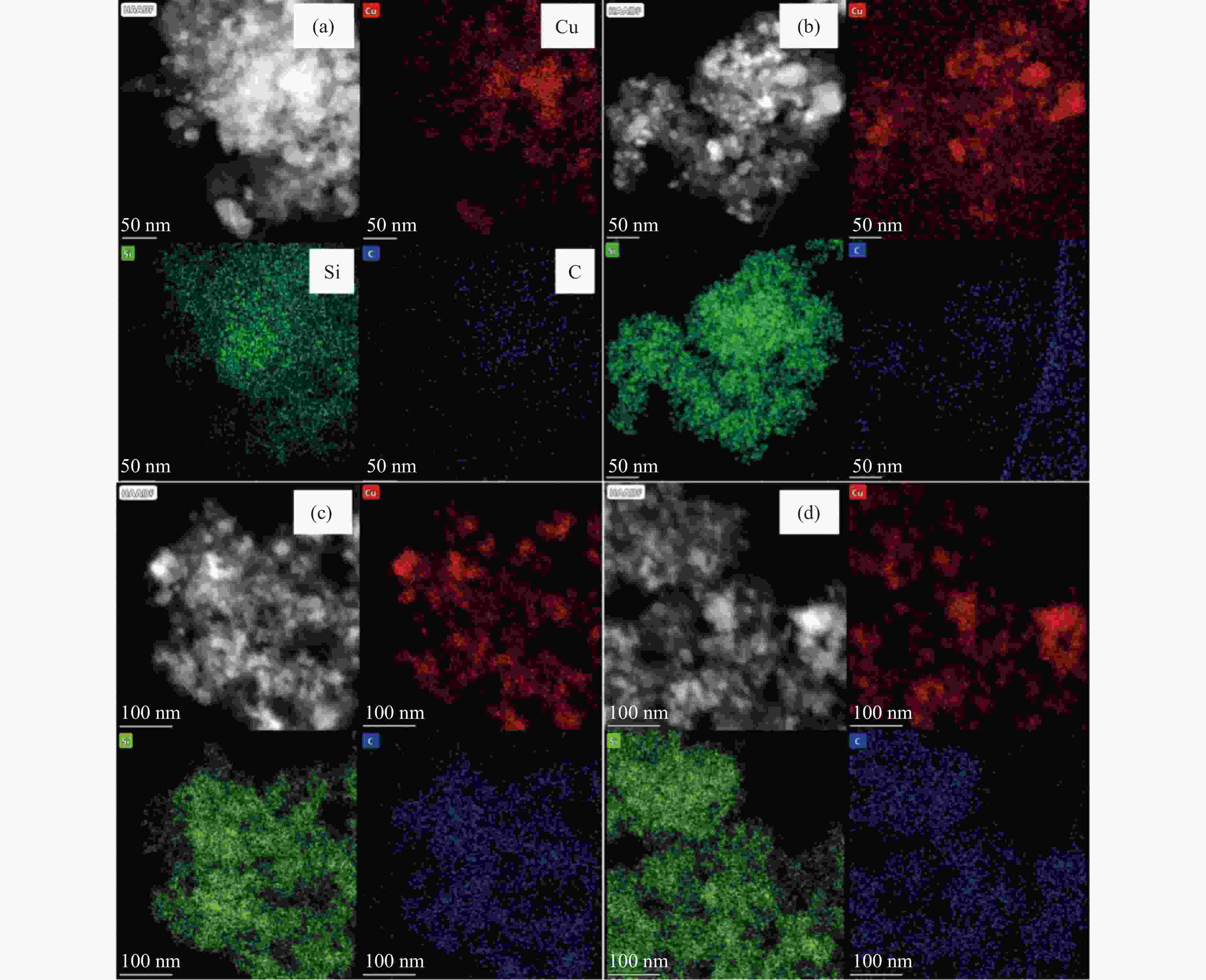
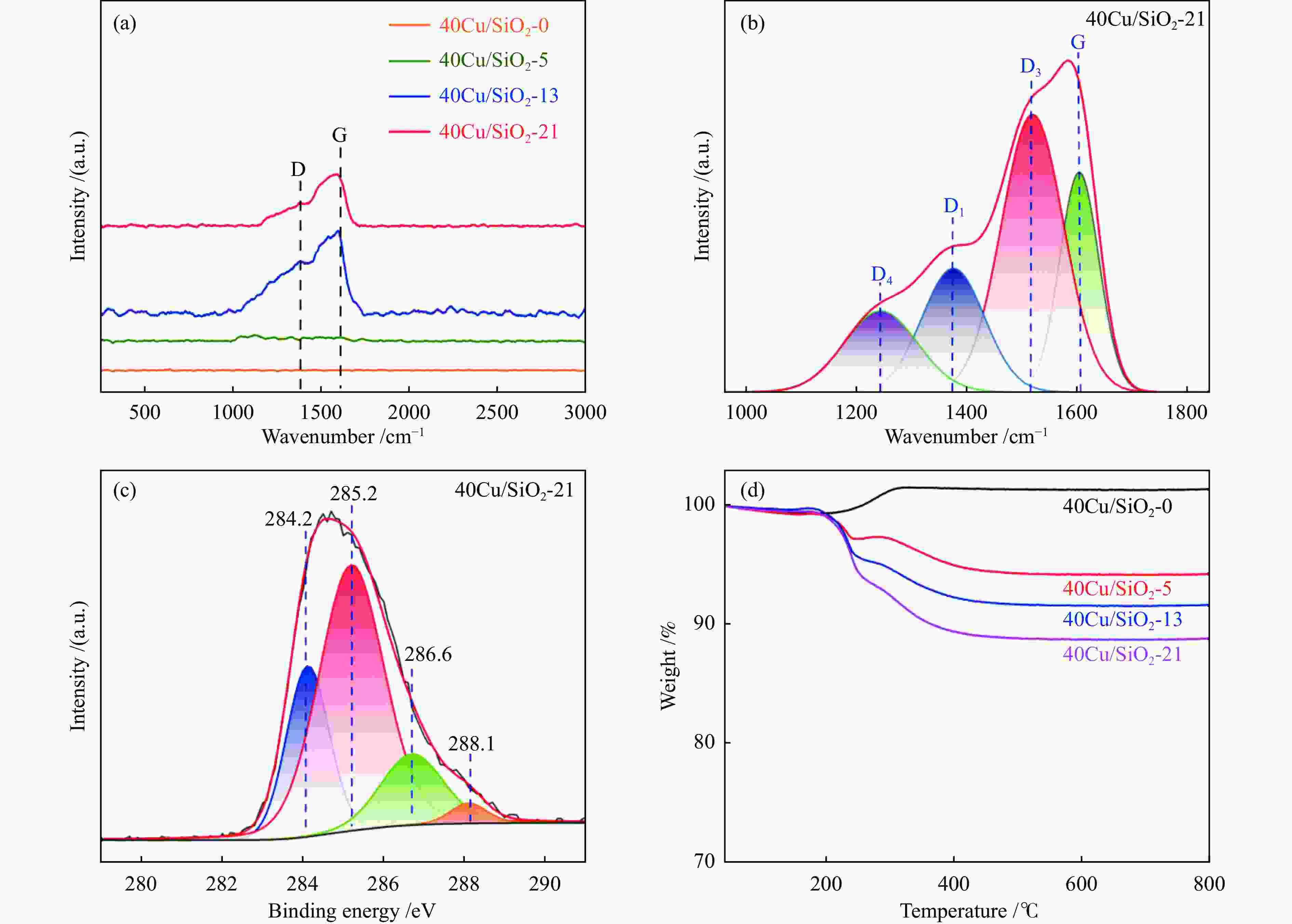
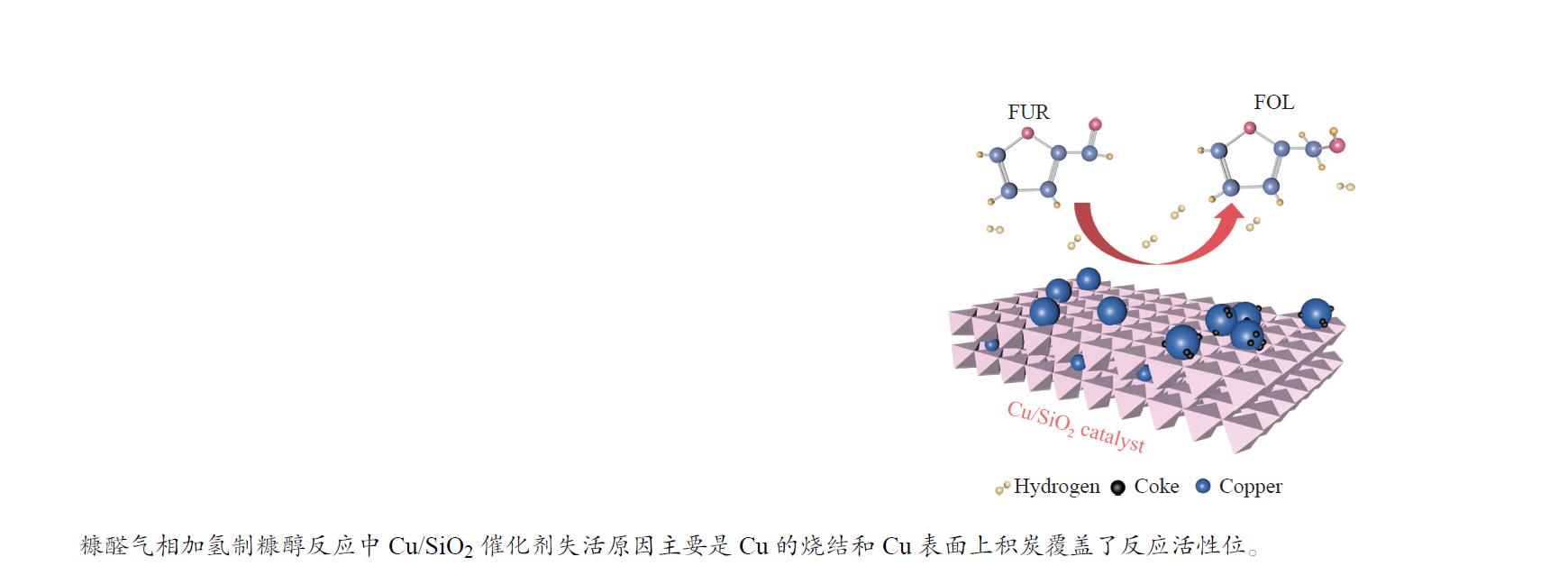
摘要: 采用共沉淀法制备了Cu/SiO2催化剂,在固定床反应器上评价其糠醛气相催化加氢制备糠醇的反应性能,并采用XRD、H2-TPR、ICP-OES、XPS、TG、Raman、TEM等手段对使用后的Cu/SiO2催化剂进行表征,研究其在反应中的失活机理。在常压、反应温度140 ℃、质量空速2.4 h−1、氢醛比9.7的条件下,反应5 h内糠醛转化率均高于97%;反应6−21 h,糠醛转化率从96%快速下降到32%,说明Cu/SiO2催化剂在糠醛加氢反应中快速失活,失活的主要原因是活性组分铜的团聚烧结和催化剂表面上积炭覆盖了反应活性位。
-
关键词:
- 共沉淀法 /
- Cu/SiO2催化剂 /
- 糠醛加氢 /
- 失活机理 /
- 糠醇
Abstract: The Cu/SiO2 catalysts were prepared by co-precipitation and tested for hydrogenation of furfural to furfuryl alcohol in a fixed bed reactor. The deactivation mechanism of the catalysts was investigated by characterization of H2-TPR, ICP-OES, XPS, TG, Raman and TEM. Under the conditions of atmospheric pressure, reaction temperature of 140 ℃, mass space velocity of 2.4 h−1 and the molar ratio of hydrogen to furfural of 9.7, the furfural conversion was higher than 97% in the first 5 h. However, the conversion of furfural decreased rapidly from 96% to 32% after 21 h of reaction, indicating that Cu/SiO2 catalyst was rapidly deactivated. The factors for the deactivation of Cu/SiO2 catalyst were the agglomeration and sintering of the active component copper. Moreover, the carbon deposition on the catalyst surface resulted in the covered active site Cu0. -
表 1 Cu/SiO2催化剂的物理化学性质
Table 1 Physicochemical properties of the Cu/SiO2 catalysts
Catalyst Cu loadinga
w /%Cu loadingb
w /%Weight lossc
w /%SBETd /
(m2·g−1)vtotald/
(cm3·g−1)dpored /nm TEM /nm Reduction
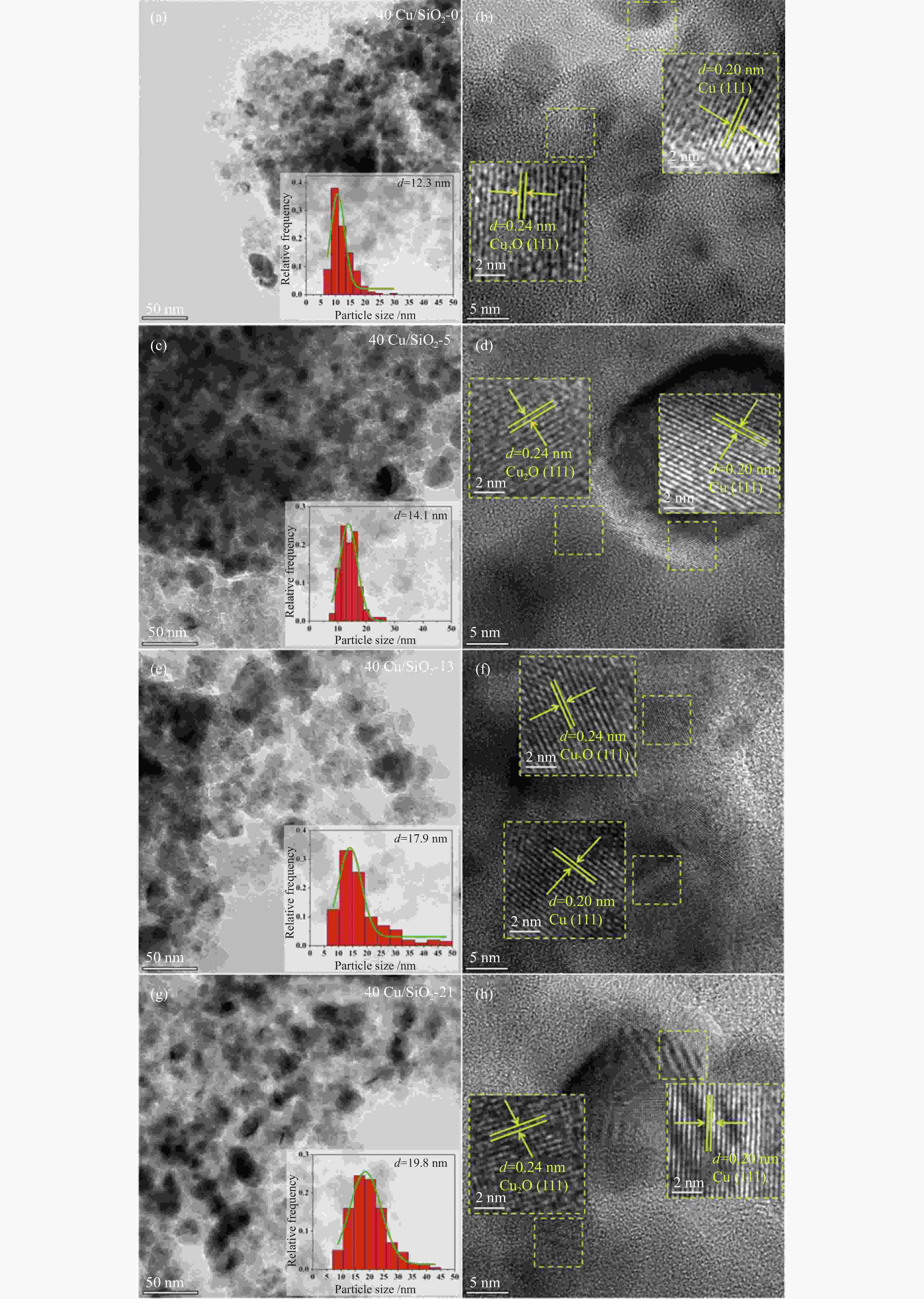
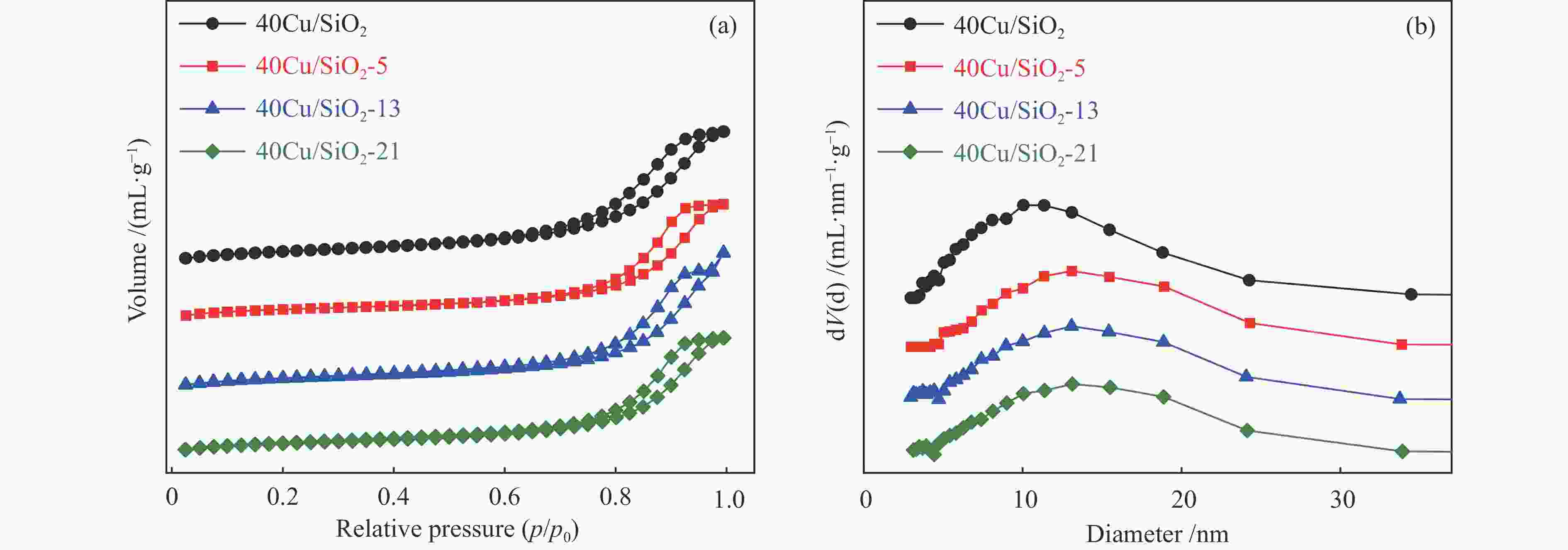
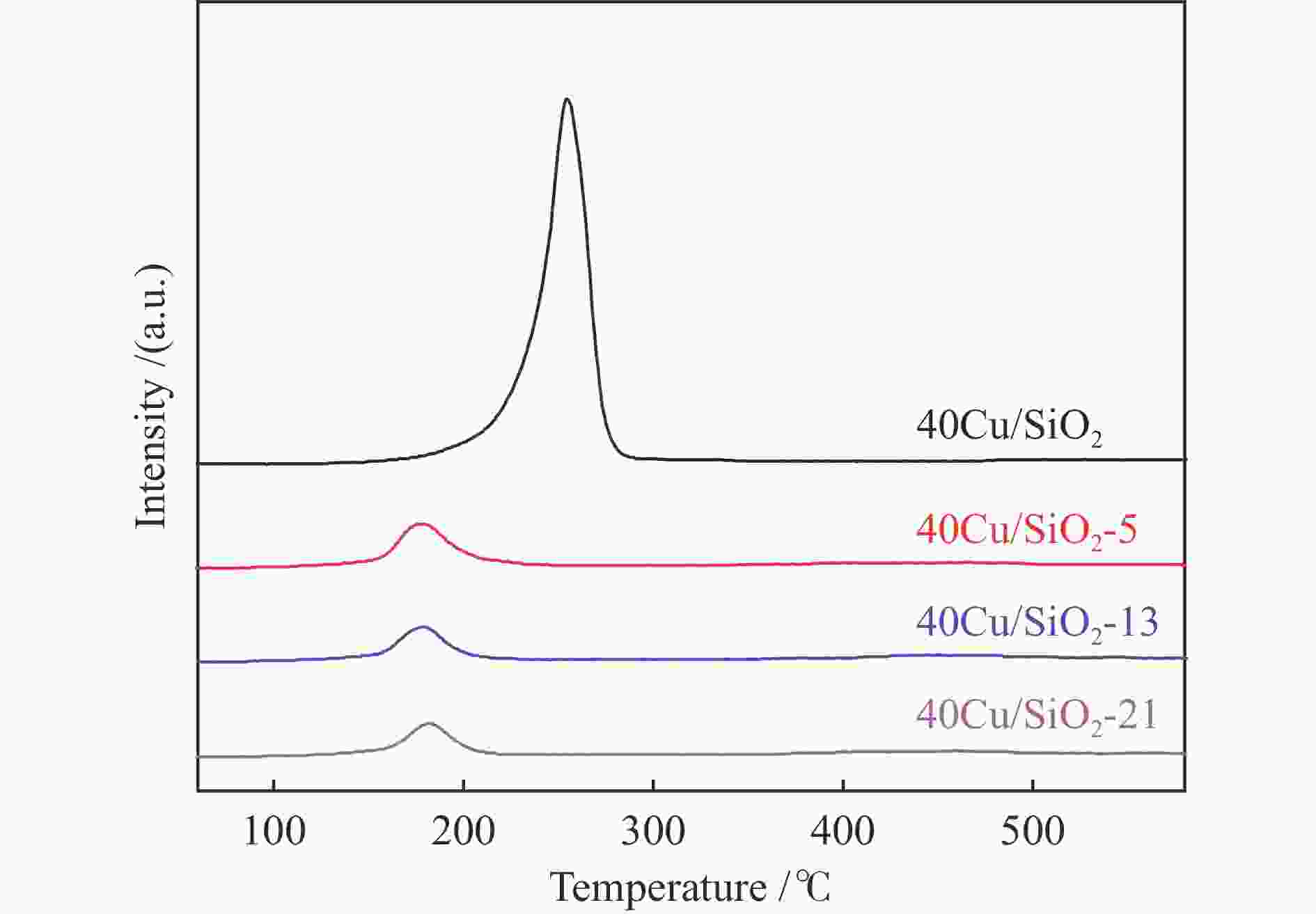
peak areae40Cu/SiO2 – – – 184 0.52 11.4 – 2210.4 40Cu/SiO2-0 30.7 – – – – – 12.3 – 40Cu/SiO2-5 29.2 30.6 5.8 142 0.45 11.1 14.1 476.3 40Cu/SiO2-13 28.6 30.4 7.5 153 0.47 13.6 17.9 369.4 40Cu/SiO2-21 26.9 30.9 11.4 139 0.45 15.6 19.8 336.6 a: copper content of the used catalysts is calculated by ICP-OES; b: copper content of the used catalysts after calcination is calculated by ICP-OES; c: detected by TG; d: determined by nitrogen adsorption; e: detected by H2-TPR 表 2 催化剂表面不同铜物种占比
Table 2 Percentage of different surface copper species
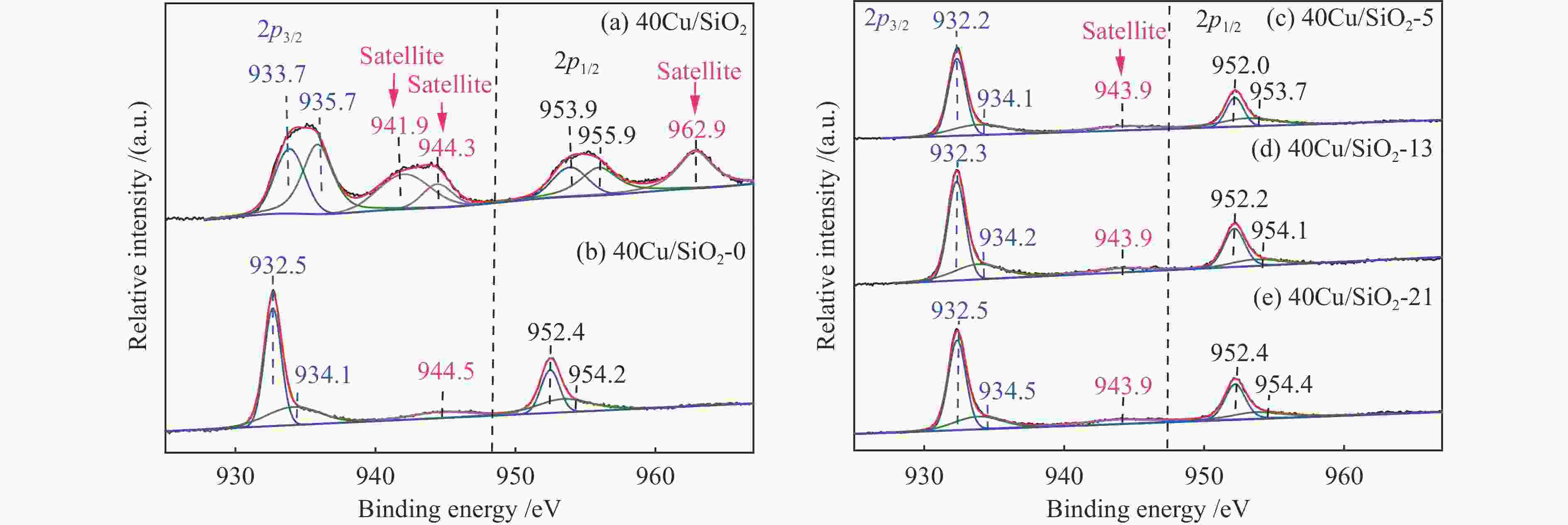
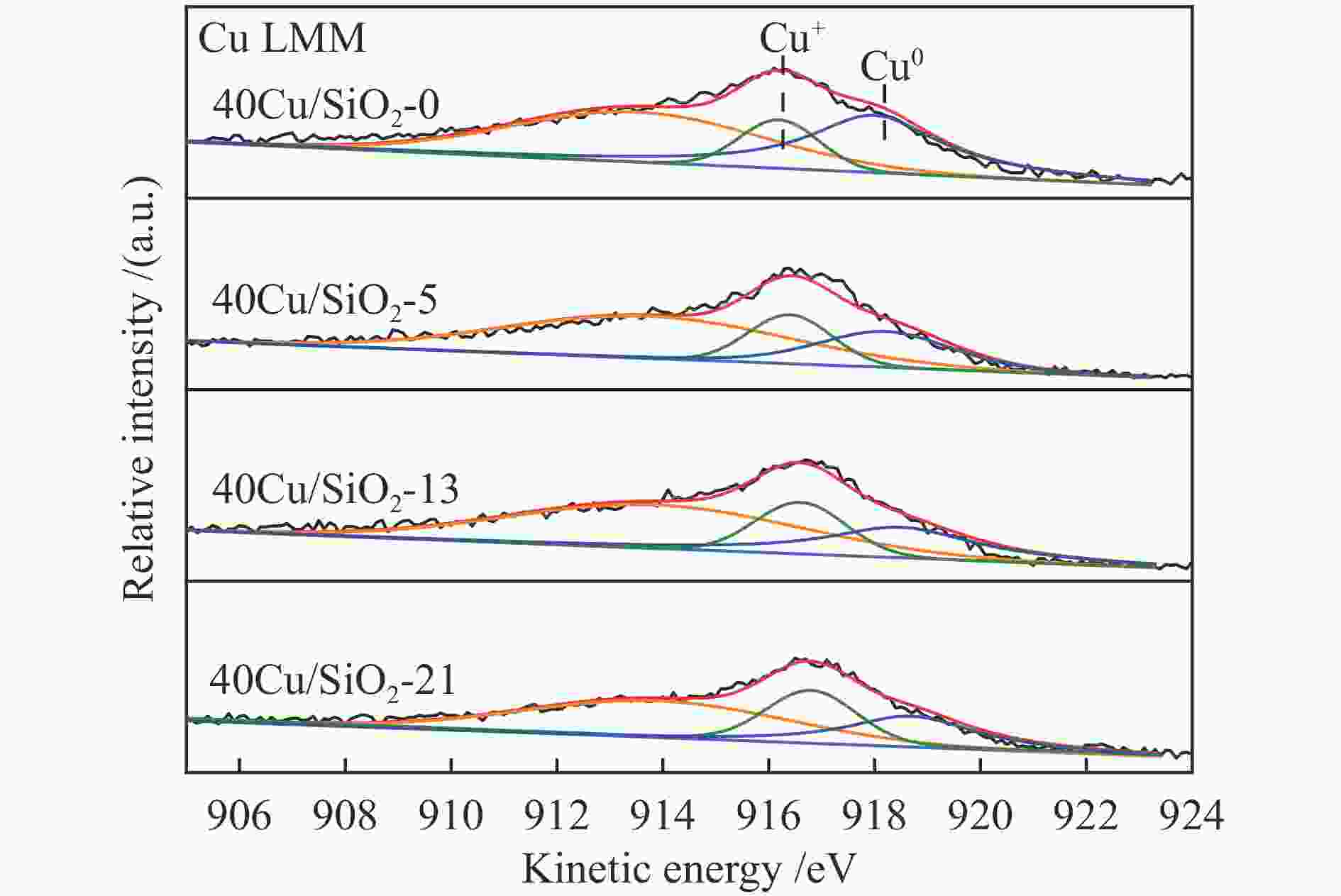
Catalysts BE /eV KE /eV x(Cu + ) /%a Cu 2p3/2 Cu + Cu0 Cu + / (Cu + + Cu0) 40Cu/SiO2 933.7 – – – 40Cu/SiO2-0 932.5 916.2 918.3 22.6 40Cu/SiO2-5 932.2 916.4 918.5 26.2 40Cu/SiO2-13 932.3 916.6 918.7 28.1 40Cu/SiO2-21 932.5 916.7 918.9 29.8 a Ratio of Cu + to (Cu + + Cu0) obtained by deconvolution of Cu LMM spectra 表 3 40Cu/SiO2-21催化剂的C 1s XPS分析
Table 3 XPS analysis of C 1s XPS of 40Cu/SiO2-21 catalyst
Binding energy /eV Chemical state of carbon Quantity /% 284.2 graphitized carbon 24.0 285.2 amorphous carbon 52.7 286.6 alcohol C–OH or C–O bond 16.7 288.1 carbonyl 6.6 -
[1] AUDEMAR M, CIOTONEA C, KARINE DE OLIVEIRA V, ROYER S, UNGUREANU A, DRAGOI B, DUMITRIU E, JEROME F. Selective hydrogenation of furfural to furfuryl alcohol in the presence of a recyclable Cobalt/SBA-15 catalyst[J]. ChemSusChem,2015,8(11):1885−1891. doi: 10.1002/cssc.201403398 [2] CHEN H, RUAN H H, LU X L, FU J, LANGRISH T, LU X Y. Efficient catalytic transfer hydrogenation of furfural to furfuryl alcohol in near-critical isopropanol over Cu/MgO-Al2O3 catalyst[J]. Mol Catal,2018,445:94−101. doi: 10.1016/j.mcat.2017.11.011 [3] YANG X H, CHEN H M, MENG Q W, ZHENG H Y, ZHU Y L, LI Y W. Insights into influence of nanoparticle size and metal-support interactions of Cu/ZnO catalysts on activity for furfural hydrogenation[J]. Catal Sci Technol,2017,7(23):5625−5634. doi: 10.1039/C7CY01284E [4] YAN K, WU G S, LAFLEUR T, JARVIS C. Production, properties and catalytic hydrogenation of furfural to fuel additives and value-added chemicals[J]. Renewable Sustainable Energy Rev,2014,38:663−676. doi: 10.1016/j.rser.2014.07.003 [5] LONG J X, ZHAO W F, XU Y F, WU W B, FANG C J, LI H, YANG S. Low-temperature catalytic hydrogenation of bio-based furfural and relevant aldehydes using cesium carbonate and hydrosiloxane[J]. RSC Adv,2019,9(6):3063−3071. doi: 10.1039/C8RA08616H [6] 朱玉雷, 李学宽, 葛世培. 糠醛气相加氢制糠醇催化反应的研究[J]. 石油化工,1992,21(7):466−469.ZHU Yu-lei, LI Xue-kuan, GE Shi-pei. A study on catalytic vapor-phase hydrogenation of furfural to furfuryl alcohol[J]. Petrochem Technol,1992,21(7):466−469. [7] 朱玉雷, 郭广庆, 苏化连, 赵让民. 糠醇的生产与催化剂[J]. 山西化工,1997,1:23−24. doi: 10.16525/j.cnki.cn14-1109/tq.1997.01.007ZHU Yu-lei, GUO Guang-qing, SU Hua-lian, ZHAO Rang-min. Production and catalyst of furfuryl alcohol[J]. Shanxi Chem Ind,1997,1:23−24. doi: 10.16525/j.cnki.cn14-1109/tq.1997.01.007 [8] GHASHGHAEE M, SHIRVANI S, GHAMBARIAN M. Kinetic models for hydroconversion of furfural over the ecofriendly Cu-MgO catalyst: An experimental and theoretical study[J]. Appl Catal A: Gen,2017,545:134−147. doi: 10.1016/j.apcata.2017.07.040 [9] LI M S, HAO Y F, CÁRDENAS-LIZANA F, KEANE M A. Selective production of furfuryl alcohol via gas phase hydrogenation of furfural over Au/Al2O3[J]. Catal Commun,2015,69:119−122. doi: 10.1016/j.catcom.2015.06.007 [10] MALIGAL-GANESH R V, XIAO C, GOH T W, WANG L L, GUSTAFSON J, PEI Y C, QI Z Y, JOHNSON D D, ZHANG S R, TAO F, HUANG W Y. A ship-in-a-bottle strategy to synthesize encapsulated intermetallic nanoparticle catalysts: Exemplified for furfural hydrogenation[J]. ACS Catal,2016,6(3):1754−1763. doi: 10.1021/acscatal.5b02281 [11] WANG Z Q, WANG X C, ZHANG C, ARAI M, ZHOU L L, ZHAO F Y. Selective hydrogenation of furfural to furfuryl alcohol over Pd/TiH2 catalyst[J]. Mol Catal,2021,508:111599. doi: 10.1016/j.mcat.2021.111599 [12] MARAKATTI V S, ARORA N, RAI S, SARMA S C, PETER S C. Understanding the role of atomic ordering in the crystal structures of nixsny toward efficient vapor phase furfural hydrogenation[J]. ACS Sustainable Chem Eng,2018,6(6):7325−7338. doi: 10.1021/acssuschemeng.7b04586 [13] P JIMENEZ-GOMEZ, CECILIA J A, D DURAN-MARTIN, MORENO-TOST R, MARISCAL, RAFAEL, MAIRELES-TORRES P. Gas-phase hydrogenation of furfural to furfuryl alcohol over Cu/ZnO catalysts[J]. J Catal,2016,336:107−115. doi: 10.1016/j.jcat.2016.01.012 [14] DU H, MA X Y, YAN P F, JIANG M, ZHAO Z, ZHANG Z C. Catalytic furfural hydrogenation to furfuryl alcohol over Cu/SiO2 catalysts: A comparative study of the preparation methods[J]. Fuel Process Technol,2019,193:221−231. doi: 10.1016/j.fuproc.2019.05.003 [15] WU J, SHEN Y M, LIU C H, WANG H B, GENG C J, ZHANG Z X. Vapor phase hydrogenation of furfural to furfuryl alcohol over environmentally friendly Cu-Ca/SiO2 catalyst[J]. Catal Commun,2005,6(9):633−637. doi: 10.1016/j.catcom.2005.06.009 [16] QING S J, HOU X N, LI L D, FENG G, WANG X, GAO Z X, FAN W B. Deactivation feature of Cu/SiO2 catalyst in methanol decomposition[J]. Int J Hydrogen Energy,2019,44(31):16667−16674. doi: 10.1016/j.ijhydene.2019.03.160 [17] JIMÉNEZ-GÓMEZ C P, CECILIA J A, MORENO-TOST R, MAIRELES-TORRES P. Selective furfural hydrogenation to furfuryl alcohol using Cu-based catalysts supported on clay minerals[J]. Top Catal,2017,60:1040−1053. [18] ZHAO Y J, KONG L, XU Y X, HUANG H J, YAO Y Q, ZHANG J W, WANG S P, MA X B. Deactivation mechanism of Cu/SiO2 catalysts in the synthesis of ethylene glycol via methyl glycolate hydrogenation[J]. Ind Eng Chem Res,2020,59(27):12381−12388. doi: 10.1021/acs.iecr.0c01619 [19] LI F, CAO B, MA R, LIANG J R, SONG H L, SONG H. Performance of Cu/TiO2-SiO2 catalysts in hydrogenation of furfural to furfuryl alcohol[J]. Can J Chem Eng,2016,94(7):1368−1374. doi: 10.1002/cjce.22503 [20] CHEN L F, GUO P J, QIAO M H, YAN S R, LI H X, WEI S, XU H L, FAN K N. Cu/SiO2 catalysts prepared by the ammonia-evaporation method: Texture, structure, and catalytic performance in hydrogenation of dimethyl oxalate to ethylene glycol[J]. J Catal,2008,257(1):172−180. doi: 10.1016/j.jcat.2008.04.021 [21] DU H, MA X Y, YAN P F, JIANG M, ZHANG Z C. Highly efficient Cu/SiO2 catalyst derived from ethanolamine modification for furfural hydrogenation[J]. Appl Catal A: Gen,2020,598:117598. doi: 10.1016/j.apcata.2020.117598 [22] 薛婧, 武朦朦, 宋有为, 赵金仙, 武建兵, 权燕红, 任军. Ag改性层状硅酸铜催化剂的草酸二甲酯加氢合成乙醇酸甲酯性能研究[J]. 燃料化学学报,2022,50(8):1014−1022. doi: 10.1016/S1872-5813(21)60011-2XUE Jing, WU Meng-meng, SONG You-wei, ZHAO Jin-xian, WU Jian-bing, QUAN Yan-hong, REN Jun. Study on performance of Ag-modified layered copper silicate catalyst for hydrogenation of dimethyl oxalate to methyl glycolate[J]. J Fuel Chem Technol,2022,50(8):1014−1022. doi: 10.1016/S1872-5813(21)60011-2 [23] CONG Y, BAO X H, ZHANG T, SUN X Y, LIANG D B. Characterization of ultrafine Cu-ZnO-ZrO2 catalysts for methanol synthesis via CO2 hydrogenation[J]. Chin J Catal,2000,21:314−318. [24] LIU D X, ZEMLYANOV D, WU T P, LOBO-LAPIDUS R J, DUMESIC J A, MILLER J T, MARSHALL C L. Deactivation mechanistic studies of copper chromite catalyst for selective hydrogenation of 2-furfuraldehyde[J]. J Catal,2013,299:336−345. doi: 10.1016/j.jcat.2012.10.026 [25] RAO R, DANDEKAR A, BAKER R T K, VANNICE M A. Properties of copper chromite catalysts in hydrogenation reactions[J]. J Catal,1997,171(2):406−419. doi: 10.1006/jcat.1997.1832 [26] MURCIA-MASCARÓS S, NAVARRO R M, GÓMEZ-SAINERO L, COSTANTINO U, NOCCHETTI M, FIERRO J L G. Oxidative methanol reforming reactions on CuZnAl catalysts derived from hydrotalcite-like precursors[J]. J Catal,2001,198(2):338−347. doi: 10.1006/jcat.2000.3140 [27] ZHENG X L, LIN H Q, ZHENG J W, DUAN X P, YUAN Y Z. Lanthanum oxide-modified Cu/SiO2 as a high-performance catalyst for chemoselective hydrogenation of dimethyl oxalate to ethylene glycol[J]. ACS Catal,2013,3(12):2738−2749. doi: 10.1021/cs400574v [28] 海雪清, 谭静静, 何静, 杨新玲, 那逸飞, 王永钊, 赵永祥. CuCo双金属催化剂催化糠醛加氢制备1, 5-戊二醇的研究[J]. 燃料化学学报,2023,51(1):1−11. doi: 10.1016/S1872-5813(22)60035-5HAI Xue-qing, TAN Jing-jing, HE Jing, YANG Xin-ling, NA Yi-fei, WANG Yong-zhao, ZHAO Yong-xiang. Hydrogenation of furfural to 1, 5-pentanediol over CuCo bimetallic catalysts[J]. J Fuel Chem Technol,2023,51(1):1−11. doi: 10.1016/S1872-5813(22)60035-5 [29] TAN J J, SU Y H, HAI X Q, HUANG L, CUI J L, ZHU Y L, WANG Y Z, ZHAO Y X. Conversion of furfuryl alcohol to 1, 5-pentanediol over CuCoAl nanocatalyst: The synergetic catalysis between Cu, CoOx and the basicity of metal oxides[J]. Mol Catal,2022,526:112391. doi: 10.1016/j.mcat.2022.112391 [30] BERNARD S, BEYSSAC O, BENZERARA K, FINDLING N, TZVETKOV G, BROWN G E. XANES, Raman and XRD study of anthracene-based cokes and saccharose-based chars submitted to high-temperature pyrolysis[J], Carbon, 2010, 48(9): 2506–2516. [31] MCGREGOR J, HUANG Z Y, PARROTT E P J, ZEITLER J A, NGUYEN K L, RAWSON J M, CARLEY A, HANSEN T W, TESSONNIER J P, SU D S, TESCHNER D, VASS E M, KNOP-GERICKE A, SCHLÖGL R, GLADDEN L F. Active coke: Carbonaceous materials as catalysts for alkane dehydrogenation[J]. J Catal,2010,269(2):329−339. doi: 10.1016/j.jcat.2009.11.016 [32] VOGELAAR B M, LANGEVELD A, EIJSBOUTS S. Analysis of coke deposition profiles in commercial spent hydroprocessing catalysts using Raman spectroscopy[J]. Fuel, 2007, 86(7/8): 1122–1129. [33] CASTANO P, ELORD G, OLAZAR M, PAWELEC B, BILBAO J. Insights into the coke deposited on HZSM-5, Hβ and HY zeolites during the cracking of polyethylene[J]. Appl Catal B: Environ,2011,104(12):91−100. [34] WANG H Z, SUN L L, SUI Z J, ZHU Y A, YE G H, CHEN D, ZHOU X G, YUAN W K. Coke formation on Pt-Sn/Al2O3 catalyst for propane dehydrogenation[J]. Ind Eng Chem Res,2018,57(26):8647−8654. doi: 10.1021/acs.iecr.8b01313 [35] HAN Z P, LI S R, JIANG F, WANG T, MA X B, GUO J L. Propane dehydrogenation over Pt-Cu bimetallic catalysts: The nature of coke deposition and the role of copper[J]. Nanoscale,2014,6(17):10000−10008. doi: 10.1039/C4NR02143F [36] ZHANG M H, TAN X C, ZHANG T, HAN Z, JIANG H X. The deactivation of a ZnO doped ZrO2-SiO2 catalyst in the conversion of ethanol/acetaldehyde to 1, 3-butadiene[J]. RSC Adv,2018,8(59):34069−34077. doi: 10.1039/C8RA06757K -




 下载:
下载: