Investigation of the promotion effect of metal oxides on the water-gas shift reaction activity over Pt-MOx/CeO2 catalysts for aqueous phase reforming
-
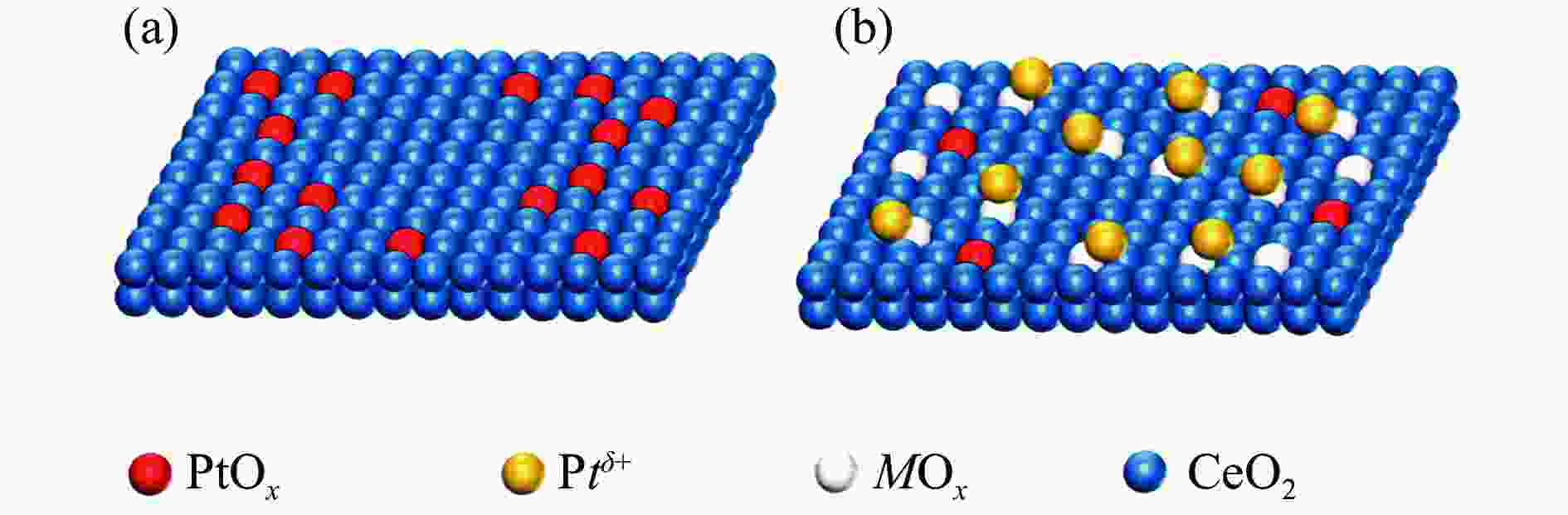
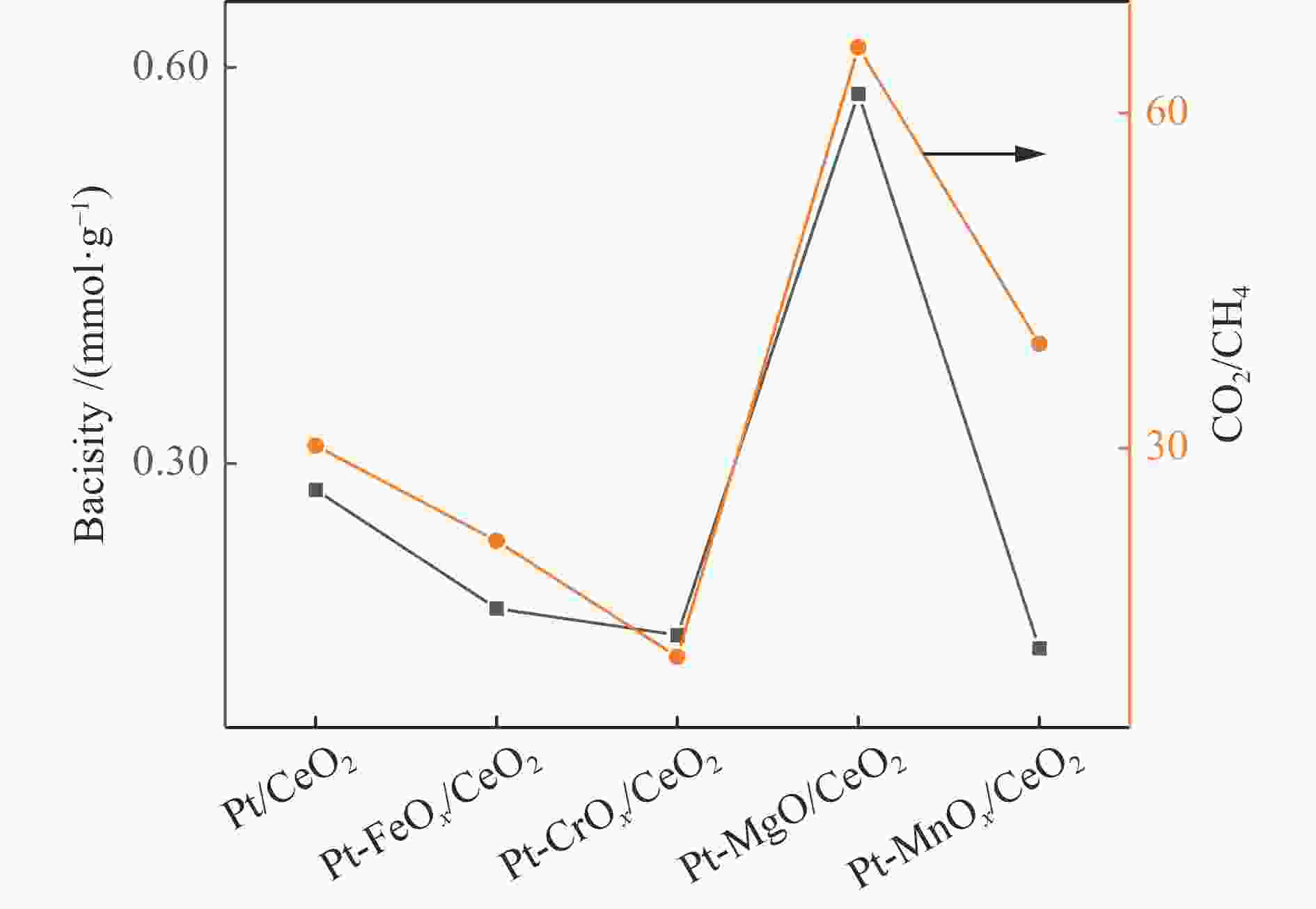
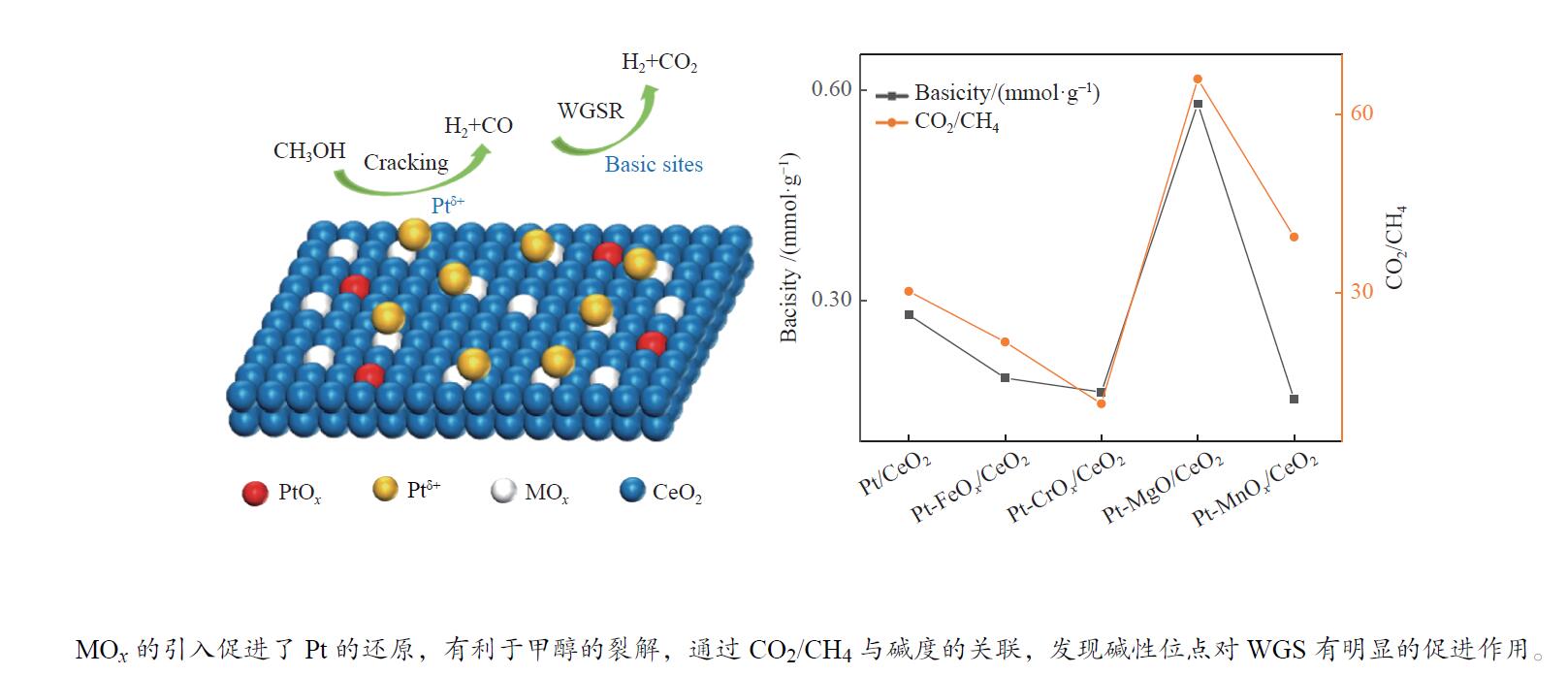
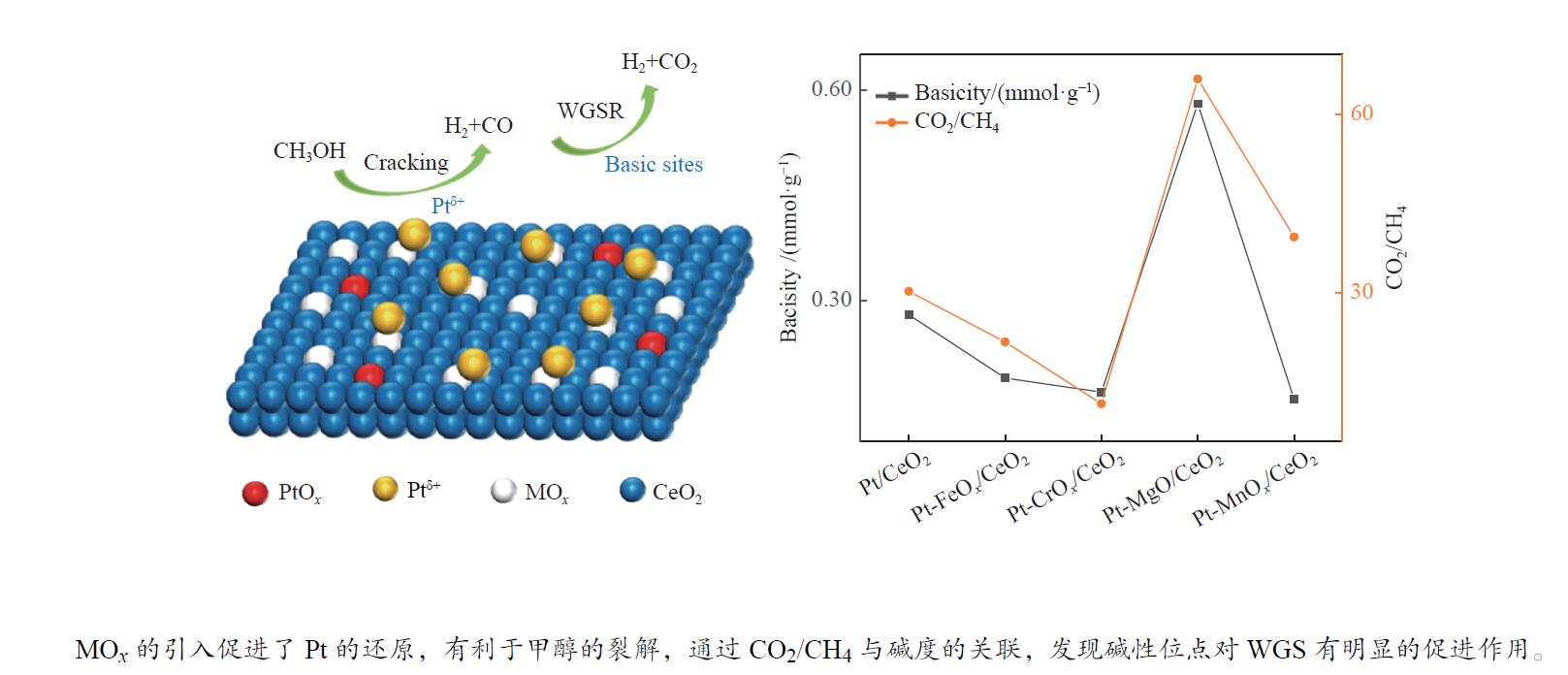
摘要: 甲醇水相重整是一种在相对温和条件下的有效产氢路径。采用分步浸渍法制备Pt/CeO2和Pt-MOx/CeO2 (M = Fe、Cr、Mg、Mn)系列催化剂,并对其反应性能进行了研究。采用XPS、XRD、TEM、CO-TPD、NH3-TPD、CO2-TPD等表征手段对催化剂的金属价态、氧空位数量、金属粒子分布、CO吸附性能和催化剂的酸/碱性等性质进行表征和分析。通过关联结果表明,MOx的加入削弱了Pt-CeO2间的相互作用,促进了价态较低的Ptδ + 的生成,这有助于C–H键的裂解,促进甲醇的转化。Pt-MgO/CeO2上的产氢量最高(164.78 mmol),CO和CH4选择性相对较低,而Pt-CrOx/CeO2上的CH4选择性最高(2.21%)。对于Pt/CeO2和Pt-MOx/CeO2 (M = Fe、Cr、Mg、Mn)催化剂的产物选择性而言,CO2/CH4比与催化剂碱度相关性较好,说明碱度促进了水分子的解离吸附和水气变换反应活性,降低了甲烷化活性。Abstract: Aqueous phase reforming (APR) of methanol is a potential pathway for the effective hydrogen production under relatively mild conditions. The Pt/CeO2 and a series of Pt-MOx/CeO2 (M = Fe, Cr, Mg, Mn) catalysts were prepared by sequential impregnation method and their APR reaction performances were studied. The catalyst properties including valence state of the promoters, the amount of oxygen vacancies, the metal distributions, the adsorption properties of CO and the acidity/basicity of catalysts were characterized and analyzed by XPS, XRD, TEM, CO-TPD, NH3-TPD, CO2-TPD, etc. It was found that the addition of MOx weakened the Pt-CeO2 interaction and promoted the generation of Ptδ + species with lower valence state, which contribute to the C−H bond cleavage and facilitate methanol conversion. The highest hydrogen production (164.78 mmol) and relatively low CO and CH4 selectivities were obtained over the Pt-MgO/CeO2, while the highest CH4 selectivity was obtained over the Pt-CrOx/CeO2 (2.21%). Over the Pt/CeO2 and Pt-MOx/CeO2 (M = Fe, Cr, Mg, Mn) catalysts, CO2/CH4 ratio correlated well with the catalyst basicity, indicating that the basicity promotes the dissociation adsorption of H2O as well as the water-gas shift (WGS) reaction activity and decreases the methanation activity.
-
Key words:
- WGS reaction /
- basicity /
- aqueous phase reforming /
- metal-support interaction
-
Table 1 APR performances over different catalysts
Catalyst Con. /% Product selectivity /% Gas yield /mmol CO2/CH4 H2 CO2 CH4 CO H2 CO2 CH4 CO Pt/CeO2 80.98 74.28 24.75 0.82 0.15 136.93 45.62 1.51 0.28 30.21 Pt-FeOx/CeO2 85.86 72.65 26.01 1.20 0.14 141.99 50.84 2.34 0.28 21.73 Pt-CrOx/CeO2 86.98 72.52 25.07 2.21 0.20 143.59 49.65 4.39 0.39 11.31 Pt-MgO/CeO2 96.77 74.80 24.74 0.38 0.08 164.78 54.51 0.83 0.18 65.67 Pt-MnOx/CeO2 76.43 72.31 26.81 0.68 0.19 125.82 46.66 1.19 0.34 39.21 Table 2 Catalyst properties of the Pt/CeO2 and Pt-MOx/CeO2 catalysts (M = Fe, Cr, Mg, Mn)
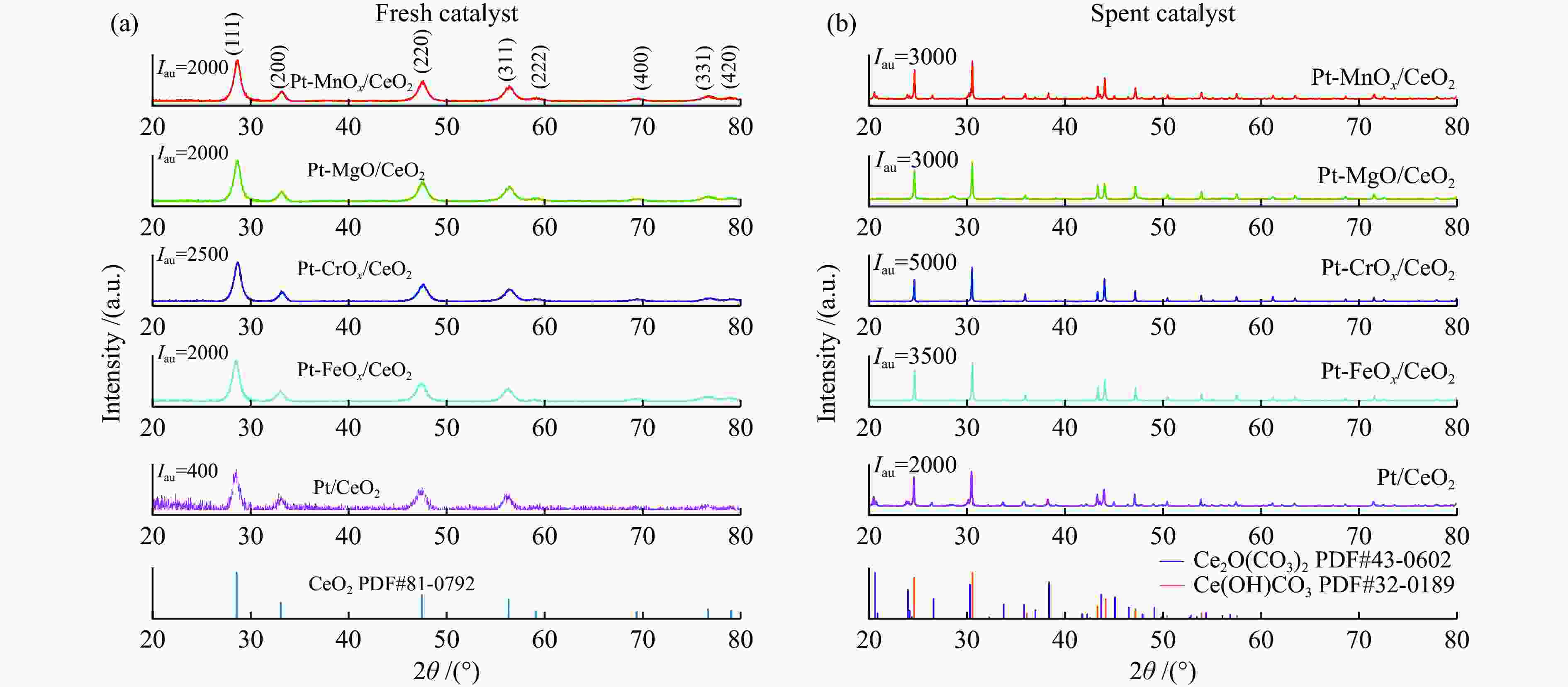
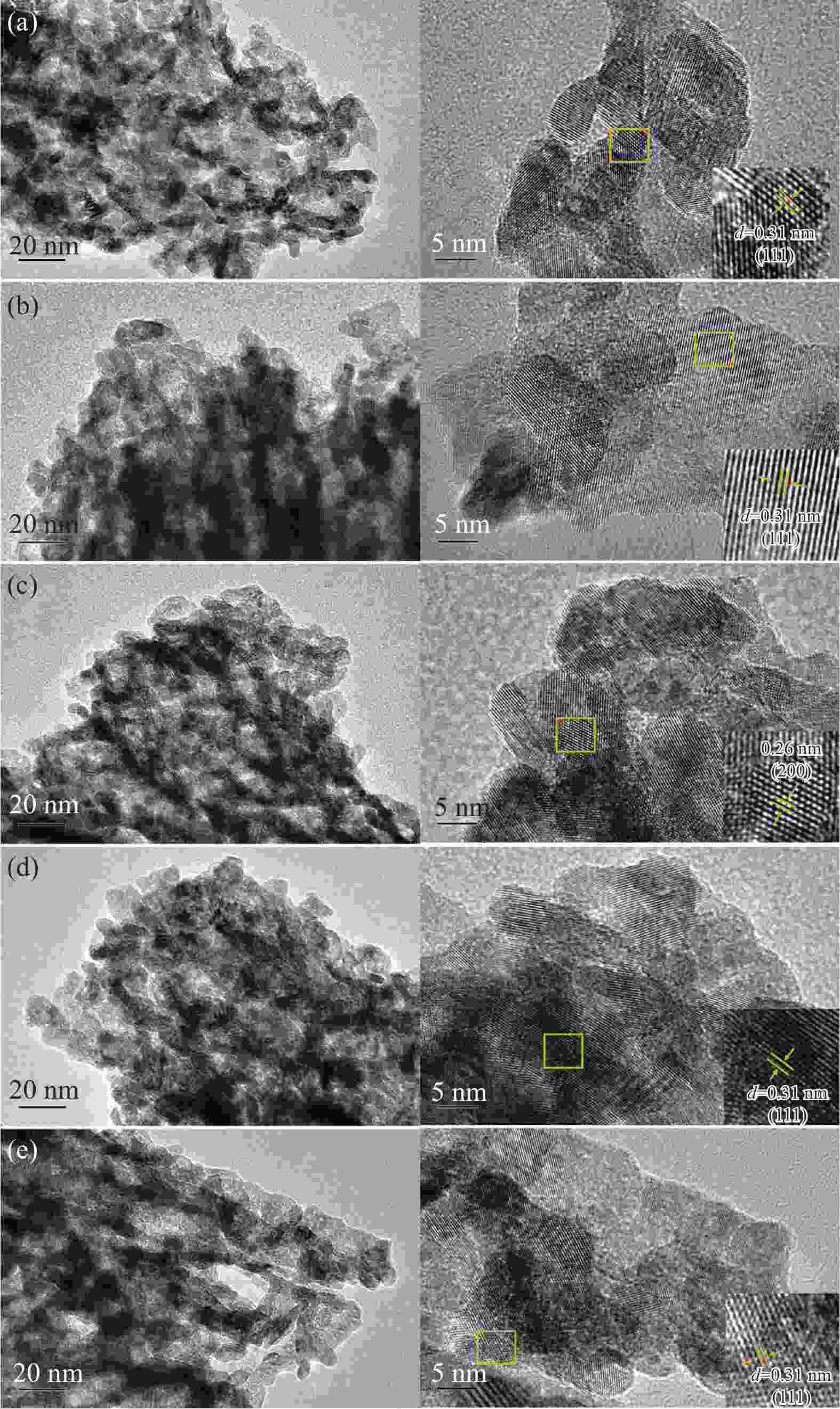
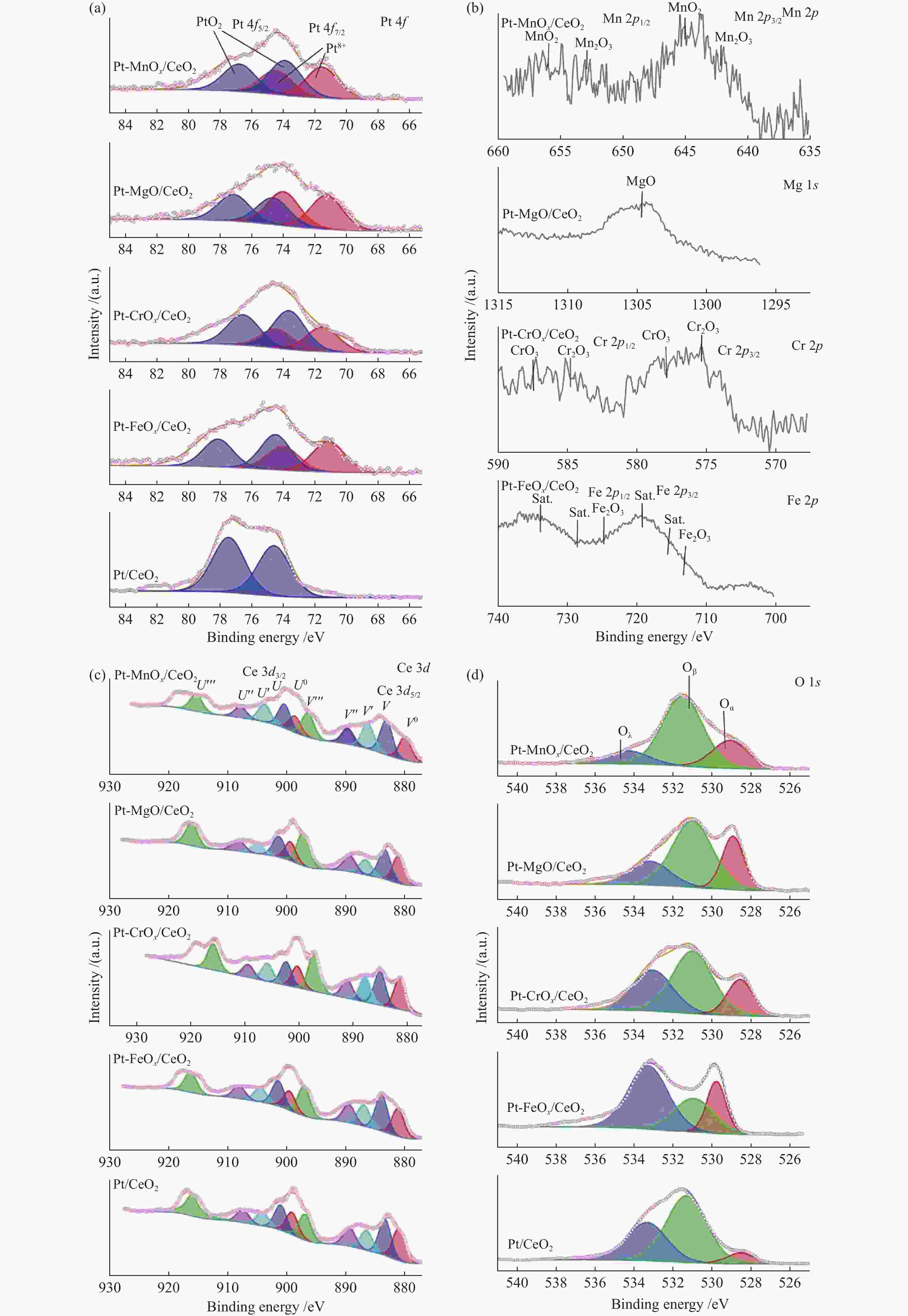
Catalyst Metal contents w/% Average diameter /nma Oβ/(Oα + Oβ + Oγ) Ce3 + /(Ce3 + + Ce4 + ) Pt M Pt/CeO2 1.74 − 9.3 57.14% 22.91% Pt-FeOx/CeO2 1.80 1.07 (Fe) 8.3 27.60% 21.76% Pt-CrOx/CeO2 1.74 1.11 (Cr) 8.0 55.56% 22.42% Pt-MgO/CeO2 1.72 0.99 (Mg) 8.5 44.84% 21.99% Pt-MnOx/CeO2 1.77 1.04 (Mn) 8.5 64.52% 39.32% a: Calculated by Scherrer equation [23] Table 3 Desorption amounts (low temperature range/high temperature range) in TPD characterizations of the Pt/CeO2 and Pt-MOx/CeO2 (M = Fe, Cr, Mg, Mn) catalysts
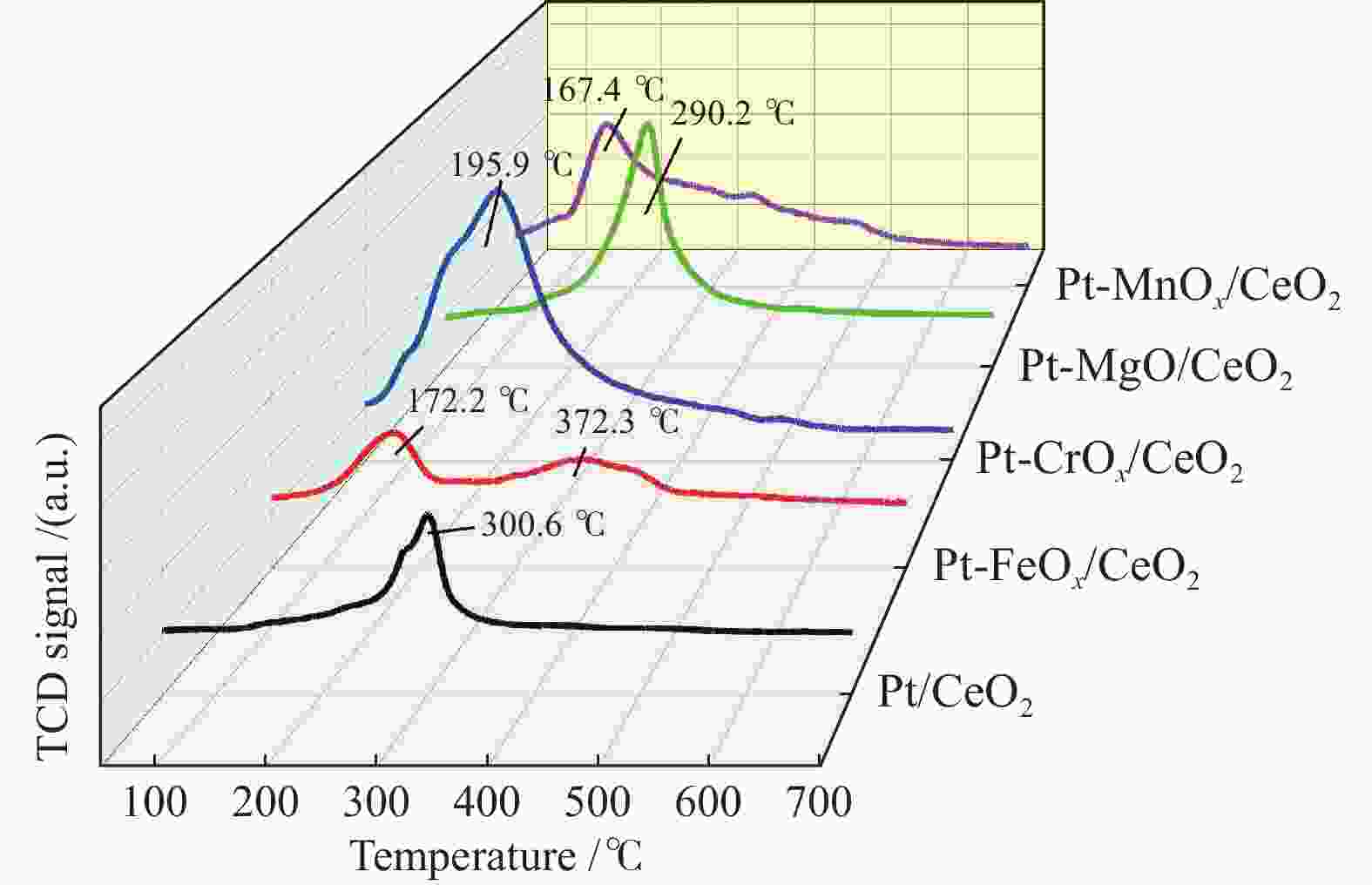
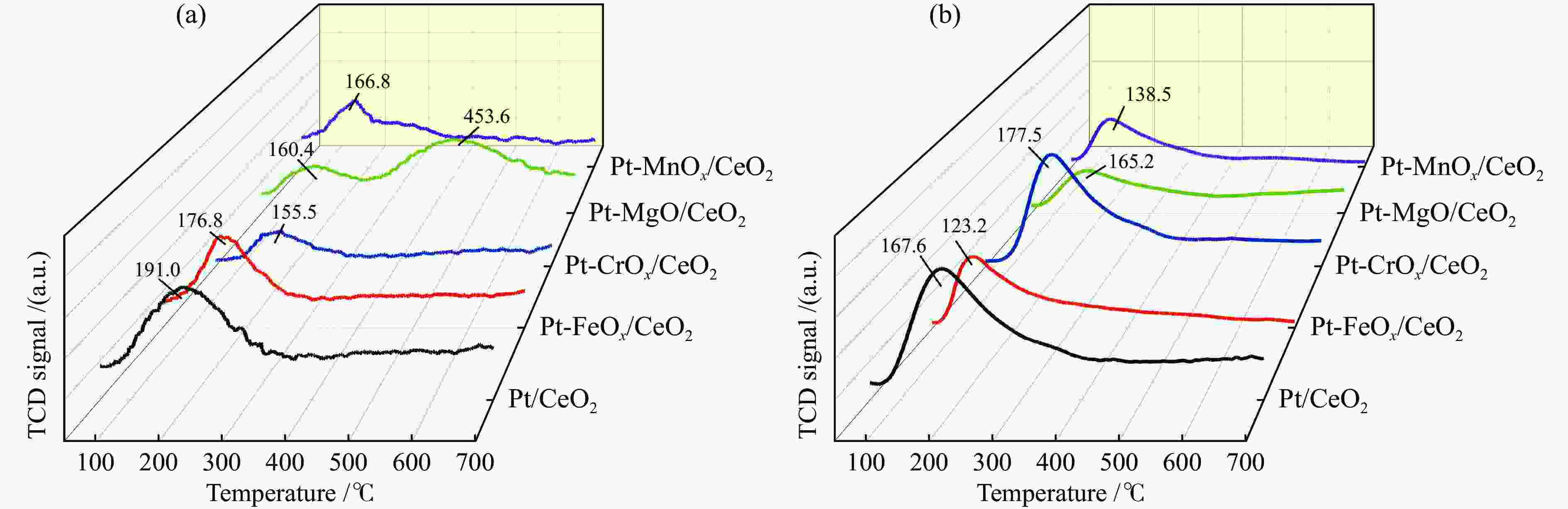
Catalyst Desorption amount /(mmol·g−1) CO-TPD CO2-TPD NH3-TPD Pt/CeO2 − /0.54 0.28/ − 1.04/ − Pt-FeOx/CeO2 0.30/0.53 0.19/ − 0.76/ − Pt-CrOx/CeO2 2.50/ − 0.17/ − 1.09/ − Pt-MgO/CeO2 − /1.37 0.07/0.51 0.43/ − Pt-MnOx/CeO2 1.99/ − 0.16/ − 0.48/ − -
[1] HOSSEINI S E, WAHID M A. Hydrogen production from renewable and sustainable energy resources: Promising green energy carrier for clean development[J]. Renewable Sustainable Energy Rev,2016,57:850−866. doi: 10.1016/j.rser.2015.12.112 [2] AHO A, ROSALES C, ERÄNEN K, SALMI T, MURZIN D, GRÉNMAN H. Biohydrogen from dilute side streams-Influence of reaction conditions on the conversion and selectivity in aqueous phase reforming of xylitol[J]. Biomass Bioenergy,2020,138:367−375. [3] OLIVEIRA A, CORDERO-LANZAC T, BAEZA J, CALVO L, HERAS F, RODRIGUEZ J, GILARRANZ M. Continuous aqueous phase reforming of a synthetic brewery wastewater with Pt/C and PtRe/C catalysts for biohydrogen production[J]. Chemosphere,2021,281:130885. doi: 10.1016/j.chemosphere.2021.130885 [4] LI C, ZHAO X, WANG A, HUBER G, ZHANG T. Catalytic transformation of lignin for the production of chemicals and fuels[J]. Chem Rev,2015,115(21):11559−11624. doi: 10.1021/acs.chemrev.5b00155 [5] SHABAKER J, DAVDA R, HUBER G, CORTRIGHT R, DUMESIC J. Aqueous-phase reforming of methanol and ethylene glycol over alumina-supported platinum catalysts[J]. J Catal,2003,215(2):344−352. doi: 10.1016/S0021-9517(03)00032-0 [6] CRUZ I, RIBEIRO N, ARANDA D, SOUZA M. Hydrogen production by aqueous-phase reforming of ethanol over nickel catalysts prepared from hydrotalcite precursors[J]. Catal Commun,2008,9(15):2606−2611. doi: 10.1016/j.catcom.2008.07.031 [7] TAO J, HOU L, YAN B, CHEN G, LI W, CHEN H, LIN F. Hydrogen production via aqueous-phase reforming of ethylene glycol over a nickel-iron alloy catalyst: Effect of cobalt addition[J]. Energy Fuels,2019,34(2):1153−1161. [8] XIE T, BODENSCHATZ C J, GETMAN R B. Insights into the roles of water on the aqueous phase reforming of glycerol[J]. React Chem Eng,2019,4(2):383−392. doi: 10.1039/C8RE00267C [9] LIN K, HUANG M, CHANG A. Liquid phase reforming of rice straw for furfural production[J]. Int J Hydrogen Energy,2013,38(35):15794−15800. doi: 10.1016/j.ijhydene.2013.06.088 [10] WEN G, XU Y, XU Z, TIAN Z. Characterization and catalytic properties of the Ni/Al2O3 catalysts for aqueous-phase reforming of glucose[J]. Catal Lett,2009,129(1):250−257. [11] GENG N, CHEN W, XU H, DING M, LIN T. Insights into the novel application of Fe-MOFs in ultrasound-assisted heterogeneous Fenton system: Efficiency, kinetics and mechanism[J]. Ultrason Sonochem,2021,72:105411. doi: 10.1016/j.ultsonch.2020.105411 [12] REYNOSO A, IRIARTE-VELASCO U, GUTIÉRREZ-ORTIZ M, AYASTUY J. Highly stable Pt/CoAl2O4 catalysts in aqueous-phase reforming of glycerol[J]. Catal Today,2021,367(21):278−289. [13] WEN G, XU Y, MA H, XU Z, TIAN Z. Production of hydrogen by aqueous-phase reforming of glycerol[J]. Int J Hydrogen Energy,2008,33(22):6657−6666. doi: 10.1016/j.ijhydene.2008.07.072 [14] MENEZES O, RODRIGUEST, ZIMMARO A, BORGES L, FRAGA M A. Production of renewable hydrogen from aqueous-phase reforming of glycerol over Pt catalysts supported on different oxides[J]. Renewable Energy,2011,36(2):595−599. doi: 10.1016/j.renene.2010.08.004 [15] LEVALLEY T L, RICHARD A R, FAN M. The progress in water gas shift and steam reforming hydrogen production technologies – A review[J]. Int J Hydrogen Energy,2014,39(30):16983−17000. doi: 10.1016/j.ijhydene.2014.08.041 [16] TAO J, ZHAO L, DONG C, LU Q, DU X, DAHLQUIST E. Catalytic steam reforming of toluene as a model compound of biomass gasification tar using Ni-CeO2/SBA-15 catalysts[J]. Energies,2013,6(7):3284−3286. doi: 10.3390/en6073284 [17] SHABAKER J, HUBER G, DAVDA R, CORTRIGHT R, DUMESIC J. Aqueous-phase reforming of ethylene glycol over supported platinum catalysts[J]. Catal Lett,2003,88:1−8. doi: 10.1023/A:1023538917186 [18] PARK Y M, SON M, PARK M-J, BAE W. Effects of Pt precursors on Pt/CeO2 to water-gas shift (WGS) reaction activity with Langmuir-Hinshelwood model-based kinetics[J]. Int J Hydrogen Energy,2020,45(51):26953−26966. doi: 10.1016/j.ijhydene.2020.06.296 [19] BRUIX A N K M, ILLAS F. Adsorption, oxidation state, and diffusion of Pt atoms on the CeO2 (111) surface[J]. J Phys Chem C,2010,114(33):14202−14207. doi: 10.1021/jp104490k [20] MAO Q, GUO Y, LIU X, SHAKOURI M, HU Y, WANG Y. Identifying the realistic catalyst for aqueous phase reforming of methanol over Pt supported by lanthanum nickel perovskite catalyst[J]. Appl Catal B: Environ,2022,313:121435−121443. doi: 10.1016/j.apcatb.2022.121435 [21] SAKAMOTO T, KIKUCHI H, MIYAO T, YOSHIDA A, NAITO S. Effect of transition metal element addition upon liquid phase reforming of methanol with water over TiO2 supported Pt catalysts[J]. Appl Catal A: Gen,2010,375(1):156−162. doi: 10.1016/j.apcata.2009.12.036 [22] ROMERO-SÁEZ M, DONGIL A B, BENITO N, ESPINOZA-GONZÁLEZ R, ESCALONA N, GRACIA F. CO2 methanation over nickel-ZrO2 catalyst supported on carbon nanotubes: A comparison between two impregnation strategies[J]. Appl Catal B: Environ,2018,237(18):817−825. [23] MANFRO R L, DA COSTA A F, RIBEIRO N F, SOUZA M M. Hydrogen production by aqueous-phase reforming of glycerol over nickel catalysts supported on CeO2[J]. Fuel Process Technol,2011,92(3):330−335. doi: 10.1016/j.fuproc.2010.09.024 [24] LI B, SU W, WANG X, WANG X. Alumina supported Ni and Co catalysts modified by Y2O3 via different impregnation strategies: Comparative analysis on structural properties and catalytic performance in methane reforming with CO2[J]. Int J Hydrogen Energy,2016,41(33):14732−14746. doi: 10.1016/j.ijhydene.2016.06.219 [25] HUANG M, FABRIS S. CO adsorption and oxidation on ceria surfaces from DFT + U calculations[J]. J Phys Chem C,2008,112(23):8643−8648. doi: 10.1021/jp709898r [26] WU S, LIU J, LIANG D, SUN H, YE Y, TIAN Z, LIANG C. Photo-excited in situ loading of Pt clusters onto rGO immobilized SnO2 with excellent catalytic performance toward methanol oxidation[J]. Nano Energy,2016,26:699−707. doi: 10.1016/j.nanoen.2016.06.038 [27] MAO A, PARK N-G, HAN G Y, PARK J H. Controlled growth of vertically oriented hematite/Pt composite nanorod arrays: Use for photoelectrochemical water splitting[J]. Nanotechnology,2011,22(17):175703. doi: 10.1088/0957-4484/22/17/175703 [28] QI F, ZHANG J, SONG L, NIU S, YANG Z, WANG Y, QIU Q. Engineering dual active sites at the interface between nanoporous Pt and nanosized CeO2 to enhance photo-thermocatalytic CO oxidation[J]. Adv Mater Interfaces,2021,8(14):2100581. doi: 10.1002/admi.202100581 [29] BEZERRA R C, MENDES P C D, PASSOS R R, DA SILVA J L. Ab initio investigation of the role of transition-metal dopants in the adsorption properties of ethylene glycol on doped Pt(100) surfaces[J]. Phys Chem,2020,22(31):17646−17658. [30] LI L-C, WANG Y-W, TIAN A-M. Adsorption of methanol on the Pt-Mo (111)/C surface[J]. Acta Phys-Chim Sin,2008,24(11):2013−2018. doi: 10.3866/PKU.WHXB20081113 [31] LIU W, LIU X, CHANG J, JIANG F, PANG S, GAO H, YU S. Efficient removal of Cr(VI) and Pb(II) from aqueous solution by magnetic nitrogen-doped carbon[J]. Front Chem Sci Eng,2021,15(5):1185−1196. doi: 10.1007/s11705-020-2032-8 [32] WANG L, YANG G, PENG S, JI D, YAN W, RAMAKRISHNA S. Fabrication of MgTiO3 nanofibers by electrospinning and their photocatalytic water splitting activity[J]. Int J Hydrogen Energy,2017,42(41):25882−25890. doi: 10.1016/j.ijhydene.2017.08.194 [33] ZHANG M, HUANG B, JIANG H, CHEN Y. Metal-organic framework loaded manganese oxides as efficient catalysts for low-temperature selective catalytic reduction of NO with NH3[J]. Front Chem Sci Eng,2017,11(4):594−602. doi: 10.1007/s11705-017-1668-5 [34] JAIN S, SHAH J, NEGI N S, SHARMA C, KOTNALA R. K. Significance of interface barrier at electrode of hematite hydroelectric cell for generating ecopower by water splitting[J]. Int J Energy Res,2019,43(9):4743−4755. doi: 10.1002/er.4613 [35] RANGASWAMY A, SUDARSANAM P, REDDY B M. Rare earth metal doped CeO2-based catalytic materials for diesel soot oxidation at lower temperatures[J]. J Rare Earth,2015,33(11):1162−1169. doi: 10.1016/S1002-0721(14)60541-X [36] SUDARSANAM P, MALLESHAM B, REDDY P S, GROßMANN D, GRÜNERT W, REDDY B M. Nano-Au/CeO2 catalysts for CO oxidation: Influence of dopants (Fe, La and Zr) on the physicochemical properties and catalytic activity[J]. Appl Catal B: Environ,2014,144:900−908. doi: 10.1016/j.apcatb.2013.08.035 [37] BAO H, CHEN X, FANG J, HUANG, W. Structure-activity Relation of Fe2O3-CeO2 composite catalysts in CO Oxidation[J]. Catalysis Letters,2008,125:160−167. doi: 10.1007/s10562-008-9540-3 [38] YAO X, MA K, ZOU W, YANG F, DONG L. Influence of preparation methods on the physicochemical properties and catalytic performance of MnO-CeO2 catalysts for NH3-SCR at low temperature[J]. Chin J Catal,2017,38(1):146−159. doi: 10.1016/S1872-2067(16)62572-X [39] TIAN Z, ZHANG W, LIU T, LIU J, WANG C. , LEI L, CHEN Y. Study on the promotion of FeOx species on water-gas shift reaction in methanol aqueous phase reforming over PtFe/Al2O3 catalyst[J]. Int J Hydrogen Energy,2022,47(98):41468−41479. doi: 10.1016/j.ijhydene.2022.03.154 [40] SU Y, FU K, ZHENG Y, JI N, SONG C, MA D, LIU Q. Catalytic oxidation of dichloromethane over Pt-Co/HZSM-5 catalyst: Synergistic effect of single-atom Pt, Co3O4, and HZSM-5[J]. Appl Catal B: Environ,2021,288:119980−119992. doi: 10.1016/j.apcatb.2021.119980 [41] KONG L N, HUANG Y R, FANG Y J. Phenol in situ hydrogenation with carbon nanotube-supported Pd catalyst[J]. Adv Mater Res,2014,934(6):60−64. [42] HAGELIN-WEAVER H. Surface science: Foundations of catalysis and nanoscience[Z]. Spr Nat BV, 2002: 575-576 [43] LI J, ZHANG C, CHENG X, QING M, XU J, WU B, LI Y. Effects of alkaline-earth metals on the structure, adsorption and catalytic behavior of iron-based Fischer-Tropsch synthesis catalysts[J]. Appl Catal A: Gen,2013,464:10−19. [44] TIAN Z, WANG C, YUE J, ZHANG X, MA L. Effect of a potassium promoter on the Fischer-Tropsch synthesis of light olefins over iron carbide catalysts encapsulated in graphene-like carbon[J]. Catal Sci Technol,2019,9(11):2728−2741. doi: 10.1039/C9CY00403C [45] GUO Y, JING Y, XIA Q, WANG Y. NbOx-based catalysts for the activation of C–O and C–C bonds in the valorization of waste carbon resources[J]. Accounts Chem Res,2022,55(9):1301−1312. doi: 10.1021/acs.accounts.2c00097 [46] GUO Y, AZMAT M U, LIU X, WANG Y, LU G. Effect of support’s basic properties on hydrogen production in aqueous-phase reforming of glycerol and correlation between WGS and APR[J]. Appl Energy,2012,92:218−223. doi: 10.1016/j.apenergy.2011.10.020 [47] TAGHAVI S, GHEDINI E, MENEGAZZO F, SIGNORETTO M, GAZZOLI D, PIETROGIACOMI D, FORNASARI G. MCM-41 supported Co-based bimetallic catalysts for aqueous phase transformation of glucose to biochemicals[J]. Processes,2020,8(7):843−858. doi: 10.3390/pr8070843 [48] DE SOUZA G, ÁVILA V C, MARCÍLIO N R, PEREZ-LOPEZ O. Synthesis gas production by steam reforming of ethanol over M-Ni-Al hydrotalcite-type catalysts; M=Mg, Zn, Mo, Co[J]. Procedia Eng,2012,42:1805−1815. doi: 10.1016/j.proeng.2012.07.575 [49] XIU P, LU X, WANG D, CHEN J, XU C, GU X. Efficient depolymerization of alkaline lignin to phenolic monomers over non-precious bimetallic Ni-Fe/CeO2-Al2O3 catalysts[J]. Biomass Convers Bior, 2022: 1−15. [50] GOMA D, DELGADO J J, LEFFERTS L, FARIA J, CALVINO J, CAUQUI M Á. Catalytic performance of Ni/CeO2/X-ZrO2 (X = Ca, Y) catalysts in the aqueous-phase reforming of methanol[J]. Nanomaterials (Basel),2019,9(11):1582−1600. doi: 10.3390/nano9111582 [51] FARPóN M G H W, PRIETO G. Supported metal single-atom thermocatalysts for the activation of small molecules[J]. Supp Metal Sin Atom Catal,2022,15(1):425−472. [52] LIU D, DOU B, ZHANG H, WU K, ZENG P, XU Y. Comparison of gelatinous and calcined magnesia supported Ni or/and Co-based catalysts for aqueous phase reforming of glycerol[J]. Renewable Energy,2022,186:656−666. doi: 10.1016/j.renene.2022.01.040 [53] HE J, WU P, LU L, LI H, JI H, HE M. Lattice-refined transition-metal oxides via ball milling for boosted catalytic oxidation performance[J]. ACS Appl Mater Inter,2019,11(40):36666−366675. doi: 10.1021/acsami.9b12063 -




 下载:
下载: