Adsorption equilibrium of methane on activated carbon and typical metal organic frameworks
-
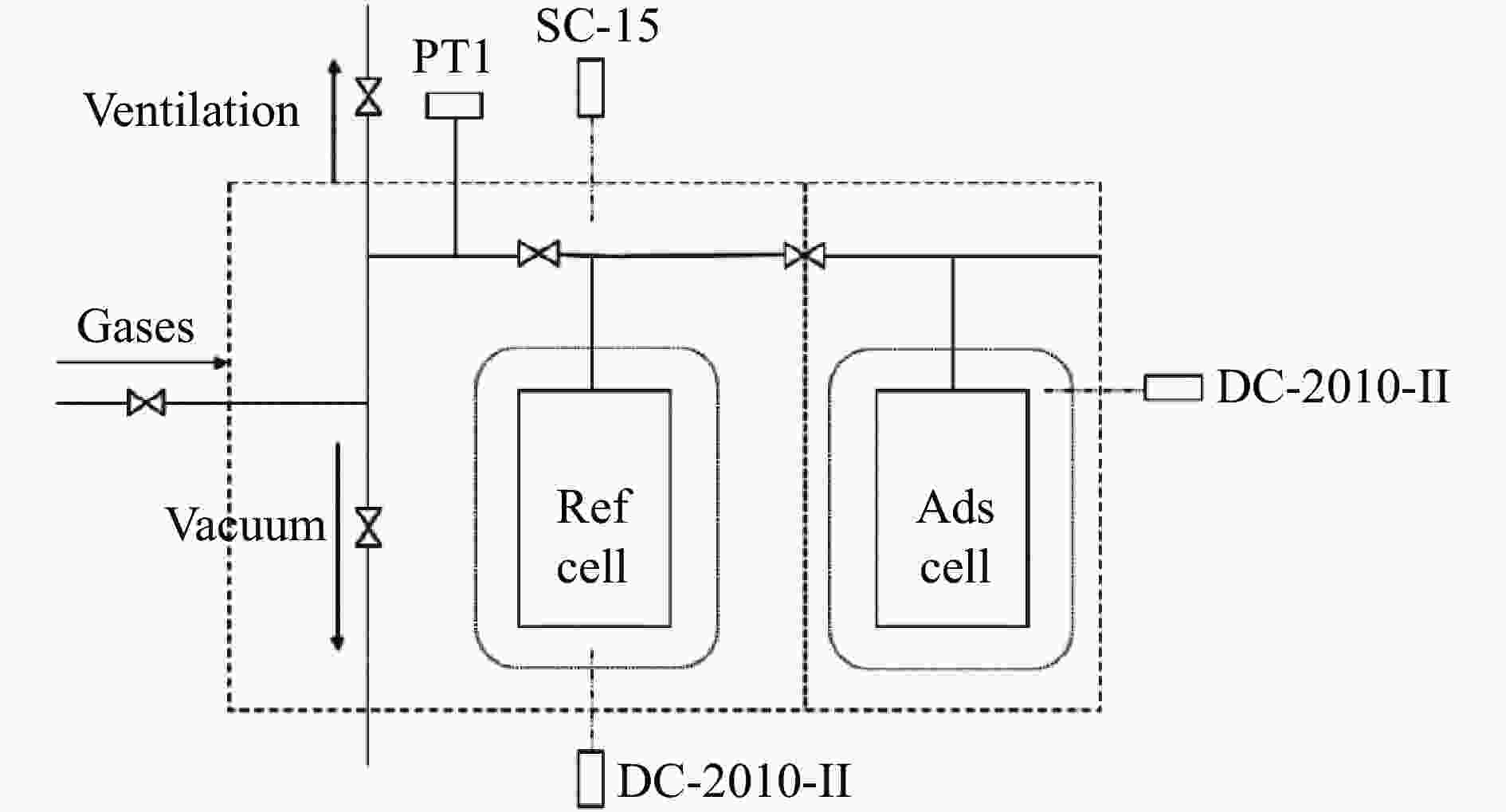
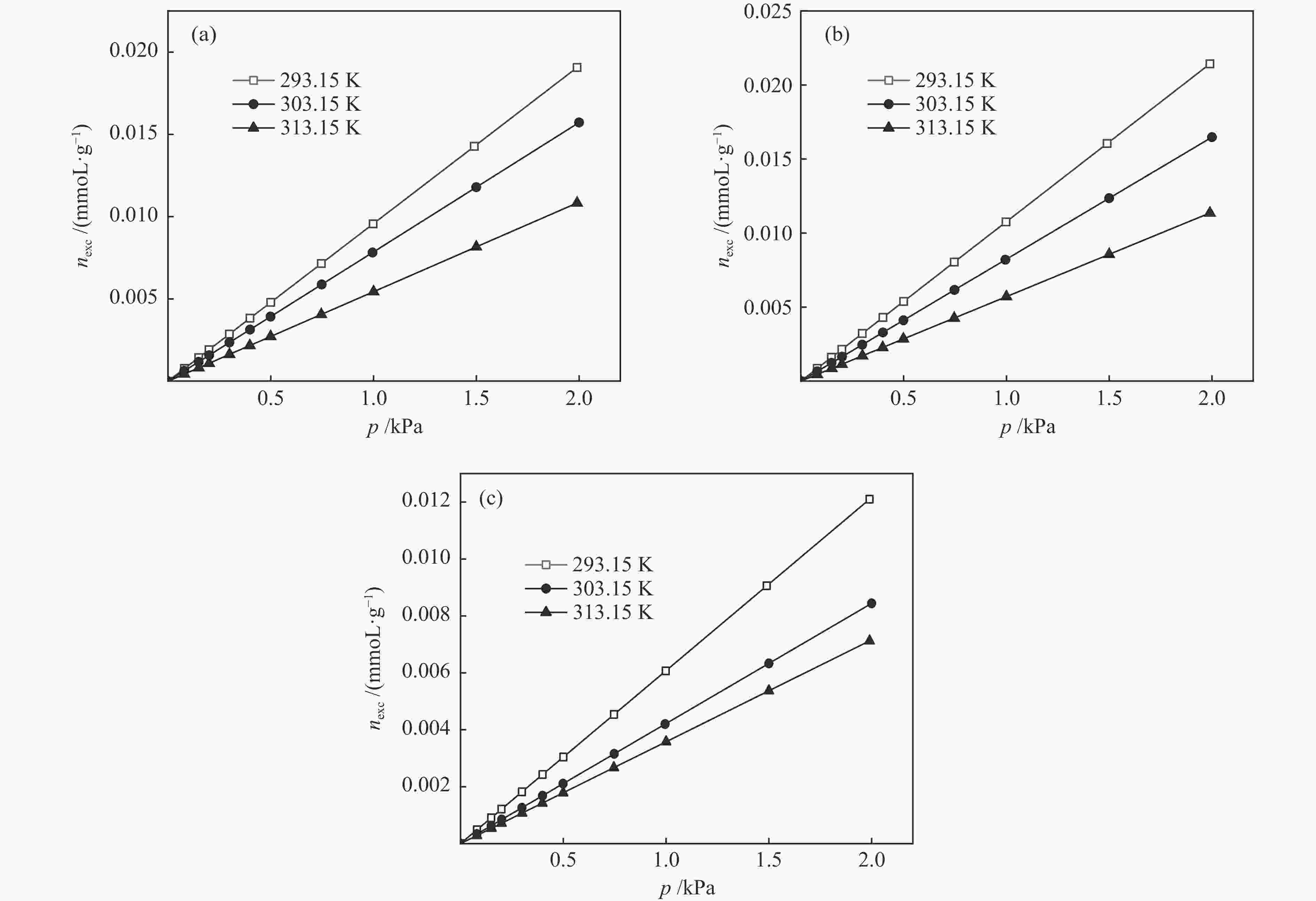
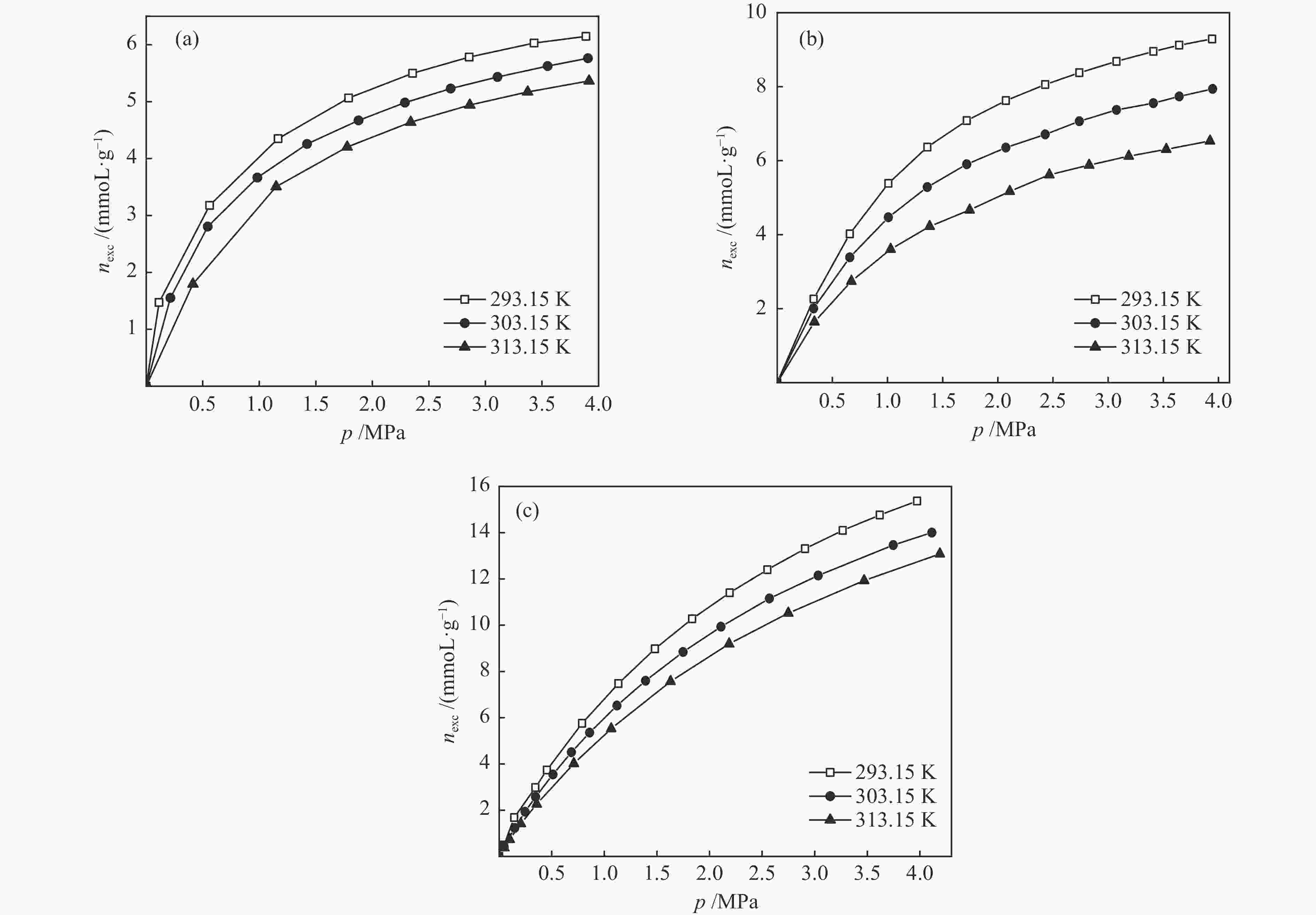
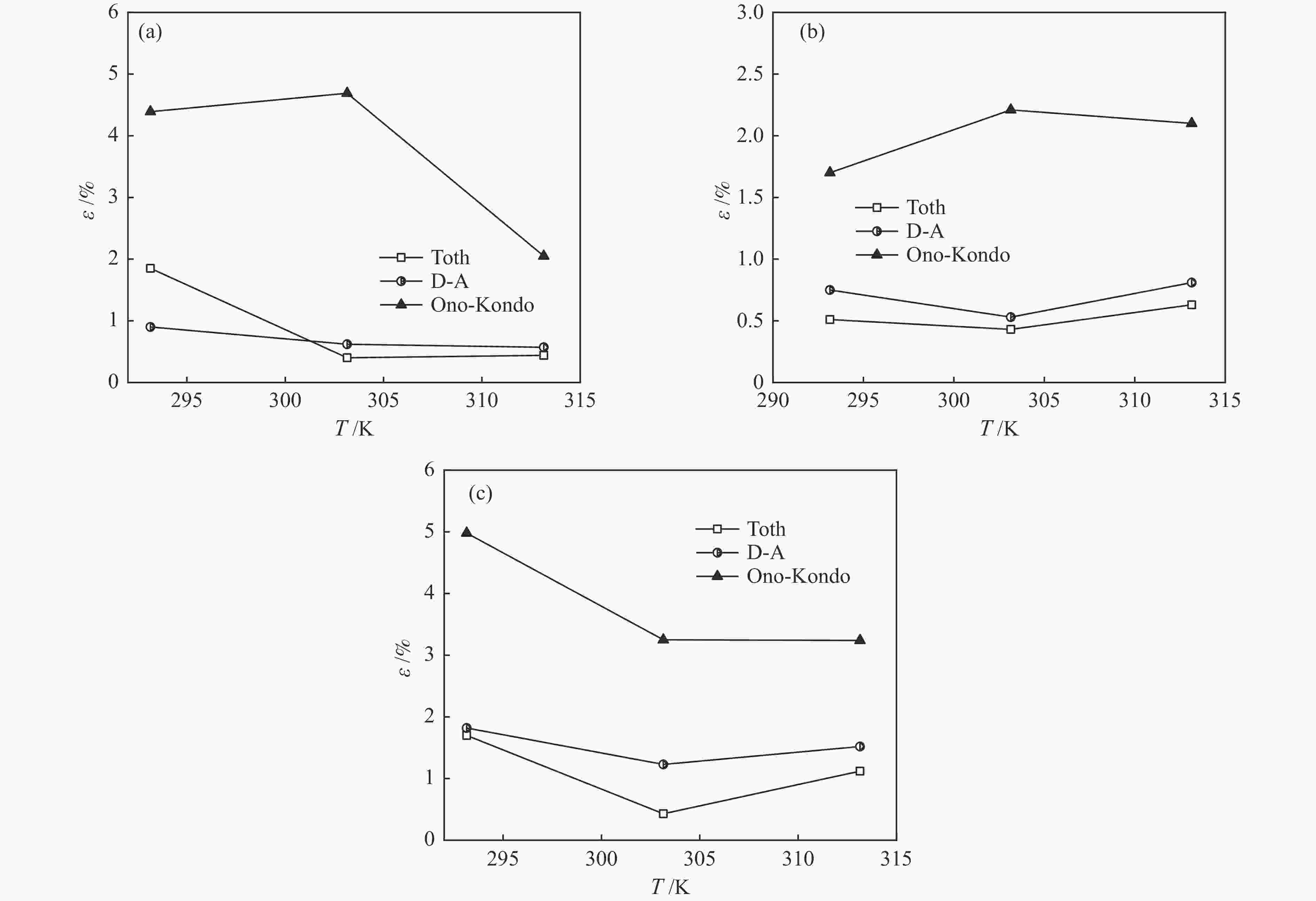
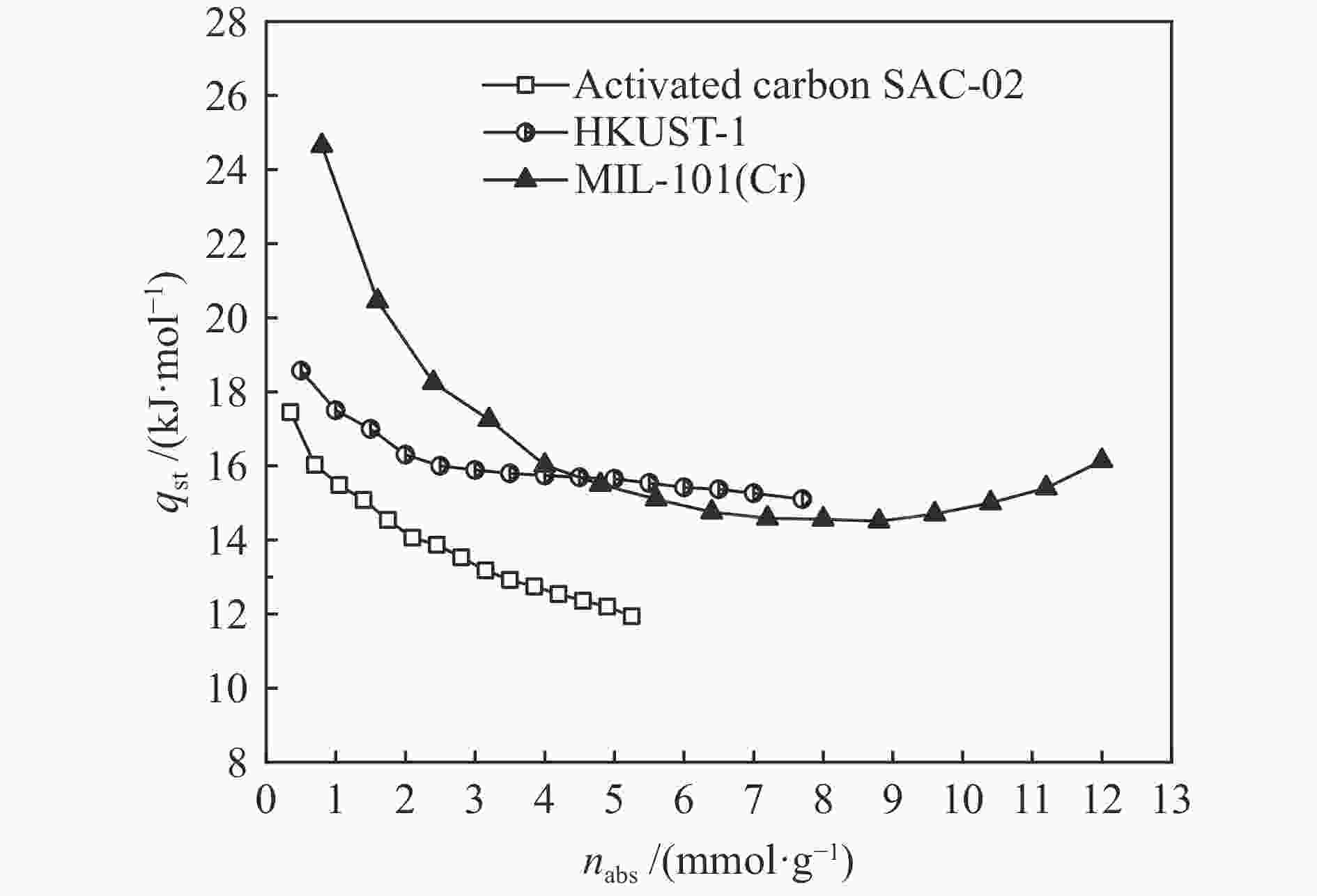
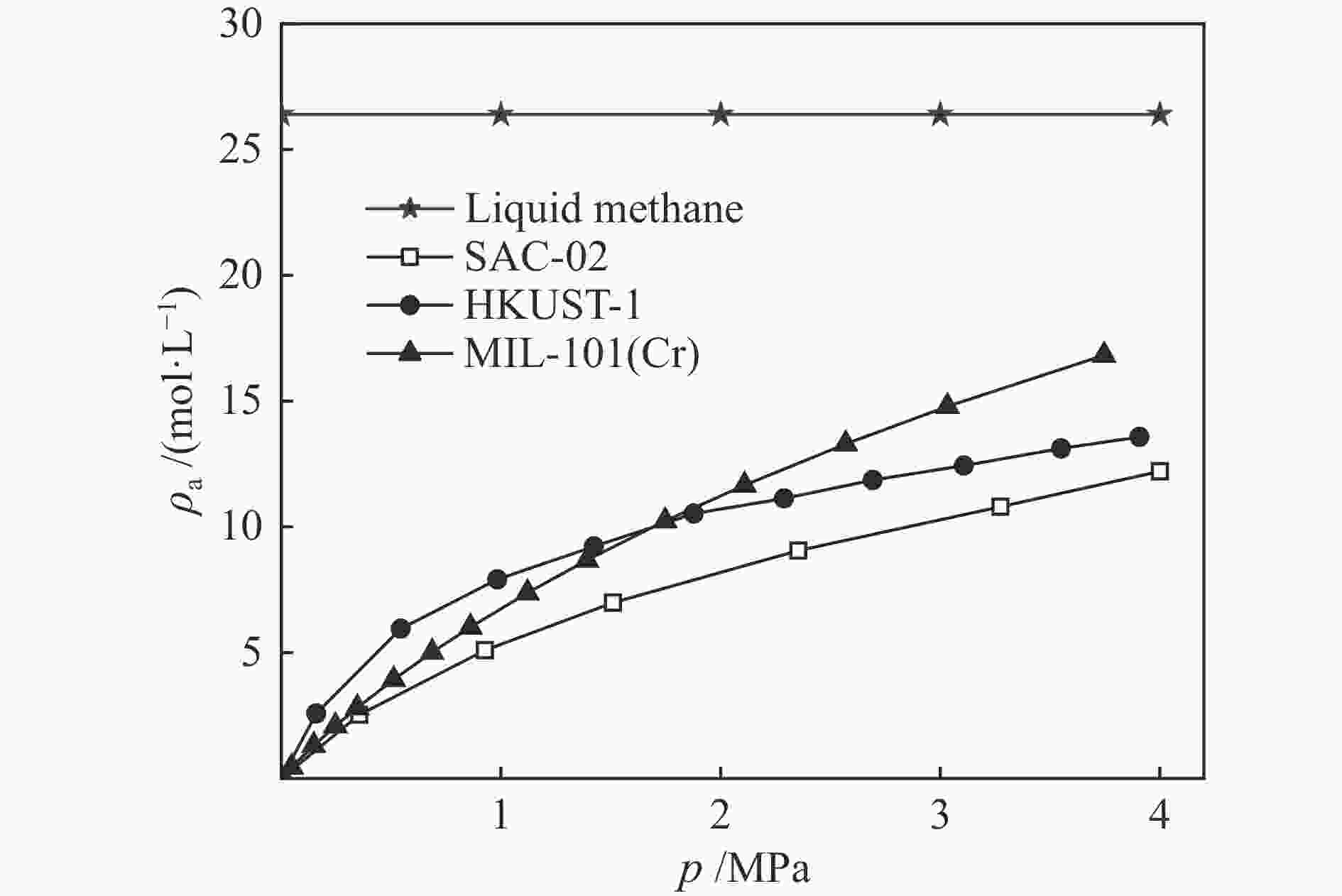
摘要: 针对吸附天然气(ANG)应用的吸附剂研发,试制了SAC-02活性炭、HKUST-1和MIL-101(Cr),通过微观形貌观察、氮气物理吸附和293.15–313.15 K、0–4 MPa条件下甲烷吸附等温线测定,采用Toth、D-A和Ono-Kondo等方程对实验数据进行关联预测比较,由等量吸附热和吸附相密度分析这些吸附剂样品对甲烷的吸附性能。结果表明,在测试范围内,Toth方程预测的吸附平衡数据精度最高,可用于ANG系统的吸附平衡分析;甲烷在MIL-101(Cr)上的平均等量吸附热最大,吸附相密度比液态甲烷的密度小但随压力的增高而增大,比活性炭和HKUST-1更适合于甲烷吸附。Abstract: To develop adsorbents suitable for the storage of natural gas by adsorption, activated carbon SAC-02, HKUST-1 and MIL-101(Cr) were synthesized and characterized in terms of structural morphology observation, nitrogen physisorption at 77.15 K, and methane adsorption at 293.15–313.15 K and 0–4 MPa. The methane adsorption isotherms were comparatively correlated with the Toth, D-A and Ono-Kondo equations and the performances of the adsorbent samples were evaluated in terms of the isosteric adsorption heat and the adsorbed phase density. The results indicate that in comparison with the D-A and Ono-Kondo equations, the Toth equation displays much smaller relative errors in correlating the methane adsorption data and is then more suitable for the adsorption equilibrium analysis on the adsorbed natural gas (ANG) system. MIL-101(Cr) exhibits the largest mean isosteric heat for methane adsorption and the density of the adsorbed methane phase is smaller than that of the liquid methane but increases with the equilibrium pressure; overall, MIL-101(Cr) with highest adsorption capacity is more suitable for methane adsorption than activated carbon and HKUST-1.
-
Key words:
- methane /
- activated carbon /
- MOFS /
- adsorption equilibrium
-
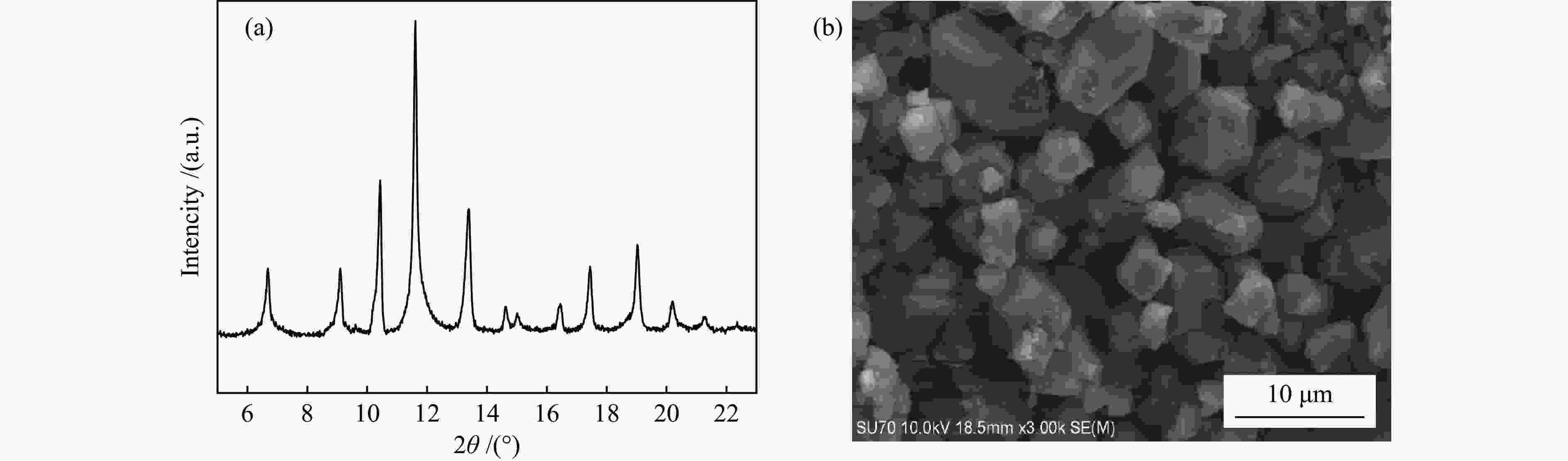
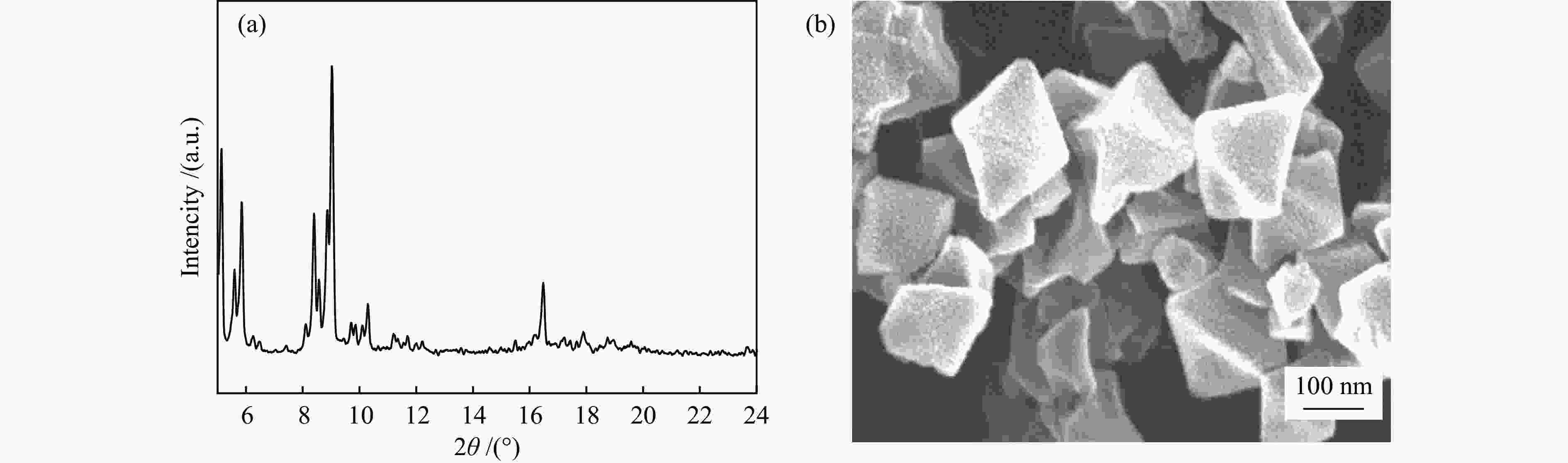
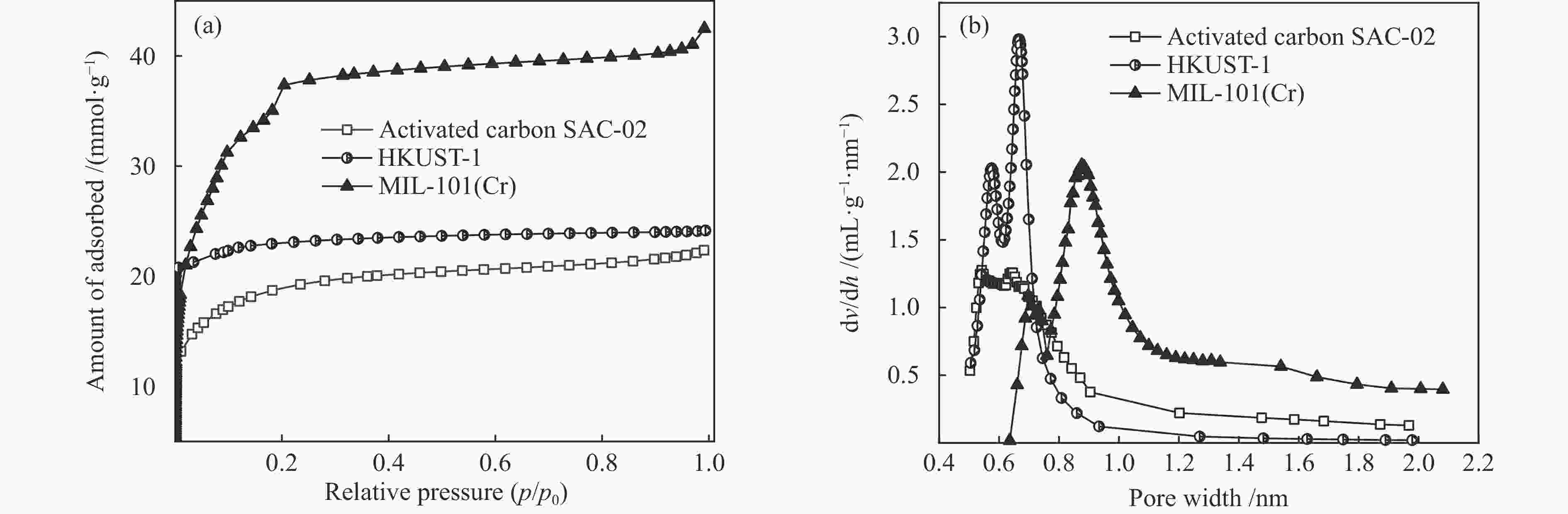
表 1 吸附剂的结构参数
Table 1 Textural properties of the samples
Sample Specific surface area /
(m2·g−1)Average pore size /
nmMicropore volume / (cm3·g−1) SAC-02 1484 1.2 0.58 HKUST-1 1850 0.70 0.77 MIL-101(Cr) 3141 1.86 1.09 表 2 甲烷在吸附剂上吸附的热力学参数
Table 2 Thermodynamic parameters of methane adsorption on the samples
Adsorbent T /
KHp /(mmol·Pa−1·g−1) qst0 /(kJ·mol−1) Isosteric adsorption heat /(kJ·mol−1) SAC-02 293.15 0.00958 23.952 24.035 303.15 0.00786 24.035 313.15 0.00544 24.118 HKUST-1 293.15 0.01077 26.601 26.685 303.15 0.00824 26.685 323.15 0.00571 26.768 MIL-101(Cr) 293.15 0.00608 29.418 29.501 303.15 0.00422 29.501 313.15 0.00358 29.584 表 3 甲烷在吸附剂上吸附平衡的Toth方程拟合参数
Table 3 Parameters fitted with the Toth equation for methane adsorption on the samples
Sample T /
Kb /
MPa−1t nm /(mmol∙g−1) va /(cm3·g−1) SAC-02 293.15 2.762 0.2426 48.01 0.7241 303.15 2.103 0.2843 42.00 0.8526 313.15 0.831 0.3161 35.85 0.8253 HKUST-1 293.15 0.837 0.2940 36.89 0.2164 303.15 0.250 0.5367 26.86 1.6300 313.15 0.546 0.6660 6.51 0.4563 MIL-101(Cr) 293.15 0.111 0.4549 141.50 1.9700 303.15 0.120 0.6155 87.85 2.5360 313.15 0.159 0.7390 50.63 1.3440 表 4 甲烷在吸附剂上吸附平衡的D-A方程拟合参数
Table 4 Parameters fitted with the D-A equation for methane adsorption on three samples
Sample T /
Kn0 /(mmol∙g−1) E /(J∙mol−1) q SAC-02 293.15 6.816 8580 1.816 303.15 6.686 8508 1.662 313.15 6.104 8501 1.931 HKUST-1 293.15 4.860 7982 1.645 303.15 4.640 7769 1.329 313.15 4.401 8420 1.707 MIL-101(Cr) 293.15 21.210 5485 1.468 303.15 18.660 5742 1.585 313.15 18.990 6112 1.614 表 5 甲烷在吸附剂上吸附平衡的Ono-Kondo方程参数
Table 5 Parameters of the Ono-Kondo equation for methane adsorption on different samples
Sample T /
K$ C $ /(mmol·g−1) $ {-(\varepsilon }_{{\rm{A}}}/k) $/
K$ {\rho }_{{\rm{mc}}} $ /(mol·L−1) SAC-02 293.15 3.995 893.24 21.03 303.15 3.670 916.75 20.51 313.15 3.769 900.70 19.99 HKUST-1 293.15 3.088 1222.06 22.52 303.15 3.030 1206.65 21.96 313.15 2.882 1225.57 21.40 MIL-101(Cr) 293.15 29.48 1242.24 24.77 303.15 13.56 1289.07 24.15 313.15 13.76 1260.73 23.54 -
[1] ALHASAN S, CARRIVEAU R, TING D S K. A review of adsorbed natural gas storage technologies[J]. Int J Environ Sci Technol,2016,73(3):343−356. [2] KUMAR K V, PREUSS K, TITIRICI M, RODRÍGUEZ-REINOSO F. Nanoporous materials for the onboard storage of natural gas[J]. Chem Rev,2017,117(3):1796−1825. doi: 10.1021/acs.chemrev.6b00505 [3] KAYAL S, SUN B, CHAKRABORTY A. Study of metal-organic framework MIL-101(Cr) for natural gas (methane) storage and compare with other MOFs (metal-organic frameworks)[J]. Energy,2015,91:772−781. doi: 10.1016/j.energy.2015.08.096 [4] ZHANG X, LIN R B, ALOTHMAN Z A, ALDUHAISH O, YILDIRIM T, ZHOU W, LI J R, CHEN B L. Promotion of methane storage capacity with metal-organic frameworks of high porosity[J]. Inorg Chem Front,2023,10(2):454−459. doi: 10.1039/D2QI02255A [5] SAHA D, FIEBACK T M, TOM B. Characteristics of methane adsorption in micro-mesoporous carbons at low and ultra-high pressure[J]. Energy Technol,2016,4(11):1392−1400. doi: 10.1002/ente.201600172 [6] SHIRAZANI M T, BAKHSHI H, RASHIDI A, TAGHIZADEH M. Starch-based activated carbon micro-spheres for adsorption of methane with superior performance in ANG technology[J]. J Environ Chem Eng,2020,8(4):103910. doi: 10.1016/j.jece.2020.103910 [7] SONG Y Q, ZHOU X L, WANG J A. Adsorption performance of activated carbon for methane with low concentration at atmospheric pressure[J]. Energ Source Part A,2021,43(11):1337−1347. doi: 10.1080/15567036.2019.1636903 [8] DENNING S, MAJID A, LUCERO J M, CRAWFORD J M, CARREON M A, KOH C A. Metal-organic framework HKUST-1 promotes methane hydrate formation for improved gas storage capacity[J]. ACS Appl Mater Interfaces,2020,12(47):53510−53518. doi: 10.1021/acsami.0c15675 [9] BIMBO N, SMITH J P, AGGARWAL H, PHYSICK A J, PUGSLEY A, BARBOUR L J, TING V P, MAYS T J. Kinetics and enthalpies of methane adsorption in microporous materials AX-21, MIL-101 (Cr) and TE7[J]. Chem Eng Res Des,2021,169:153−164. doi: 10.1016/j.cherd.2021.03.003 [10] TSIVION E, HEAD-GORDON M. Methane storage: molecular mechanisms underlying room temperature adsorption in Zn4O(BDC)(3) (MOF-5)[J]. J Phys Chem C,2017,121(22):12091−12100. doi: 10.1021/acs.jpcc.7b04246 [11] HU B, CHENG Y P, WANG L, ZHANG K Z, HE X X, YI M H. Experimental study on influence of adsorption equilibrium time on methane adsorption isotherm and Langmuir parameter[J]. Adv Powder Technol,2021,32(11):4110−4119. doi: 10.1016/j.apt.2021.09.015 [12] ZHOU L, ZHOU Y. Linearization of adsorption isotherms for high-pressure applications[J]. Chem Eng Sci,1998,53(14):2531−2536. doi: 10.1016/S0009-2509(98)00065-7 [13] RAHMAN K A, CHAKRABORTY A, SAHA B B, NG K C. On thermodynamics of methane carbonaceous materials adsorption[J]. Int J Heat Mass Transf,2012,55(4):565−573. doi: 10.1016/j.ijheatmasstransfer.2011.10.056 [14] 郑青榕, BIRKETT G, DO D D. 甲烷在活性炭上吸附的实验及理论分析[J]. 天然气化工(C1化学与化工),2009,34(1):41−45.ZHENG Qing-rong, BIRKETT G, DO D D. Experimental and theoretical analysis of methane adsorption on activated carbon[J]. Nat Gas Ind,2009,34(1):41−45. [15] 朱子文, 郑青榕, 冯玉龙, 曾斌. 甲烷在活性炭上的吸附平衡研究[J]. 天然气化工(C1化学与化工),2015,40(2):39−43.ZHU Zi-wen, ZHENG Qing-rong, FENG Yu-long, ZENG Bin. Study on the adsorption equilibrium of methane on activated carbon[J]. Nat Gas Ind,2015,40(2):39−43. [16] 张维东, 郑青榕, 王泽浩, 张轩. 甲烷在MOF-5和MOF-199上的吸附平衡[J]. 天然气化工(C1化学与化工),2019,44(6):45−51.ZHANG Wei-dong, ZHENG Qing-rong, WANG Ze-hao, ZHANG Xuan. Adsorption equilibria of methane on MOF-5 and MOF-199[J]. Nat Gas Ind,2019,44(6):45−51. [17] 廖圣平, 郑青榕, 仵梦博, 张轩. 甲烷在MIL-101(Cr)和AX-21上吸附平衡的比较分析[J]. 天然气化工(C1化学与化工),2021,46(5):81−87.LIAO Sheng-ping, ZHENG Qing-rong, WU Meng-bo, ZHANG Xuan. Comparative analysis of methane adsorption equilibria on MIL-101(Cr) and AX-21[J]. Nat Gas Ind,2021,46(5):81−87. [18] 解晨, 郑青榕. 甲烷在活性炭上的超临界温度吸附实验及理论分析[J]. 天然气化工(C1化学与化工),2012,37(1):40−44.XIE Chen, ZHENG Qing-rong. Experimental and theoretical analysis of supercritical temperature adsorption of methane on activated carbon[J]. Nat Gas Ind,2012,37(1):40−44. [19] 张英, 马蕊英, 赵亮, 王海洋, 孙兆松. 金属有机骨架材料HKUST-1的制备及其甲烷吸附性能[J]. 石油化工,2017,46(7):884−887.ZHANG Yin, MA Rui-ying, ZHAO Liang, WANG Hai-yang, SUN Zhao-song. Preparation of metal-organic skeleton material HKUST-1 and its methane adsorption performance[J]. Petrk Chen Technol,2017,46(7):884−887. [20] YU Z W, DESCHAMPS J, HAMON L, PRABHAKARAN P K, PRE P. Hydrogen adsorption and kinetics in MIL-101(Cr) and hybrid activated carbon-MIL-101(Cr) materials[J]. Int J Hydrogen Energy,2017,42(12):8021−8031. doi: 10.1016/j.ijhydene.2017.02.192 [21] FEREY G, MELLOT-DRAZNIEKS C, SERRE C, MILLANGE F, DUTOUR J, SURBLE S, MARGIOLAKI I. A chromium terephthalate-based solid with unusually large pore volumes and surface area[J]. Science,2005,309(5743):2040−2042. doi: 10.1126/science.1116275 [22] CLARK A. The Theory of Adsorption and Catalysis[M]. New York: Academic Press, 1970: 160–271. [23] MEEKS O R, RYBOLT T R. Correlations of adsorption energies with physical and structural properties of adsorbate molecules[J]. J Colloid Interface Sci,1997,196(1):103−109. doi: 10.1006/jcis.1997.5198 [24] MENON P G. Adsorption at high pressures[J]. Chem Rev,1968,68(3):277−294. doi: 10.1021/cr60253a002 [25] LI M, GU A Z, LU X S, WANG R S. Determination of the adsorbate density from supercritical gas adsorption equilibrium data[J]. Carbon,2003,41(3):585−588. doi: 10.1016/S0008-6223(02)00356-1 [26] SOAVE G. Equilibrium constants from a modified Redlich-Kwong equation of state[J]. Chem Eng Sci,1972,27(6):1197−1203. doi: 10.1016/0009-2509(72)80096-4 [27] LI J, CHEN Z, WU K, WANG K, LUO J, FENG D, QU S, LI X. A multi-site model to determine supercritical methane adsorption in energetically heterogeneous shales[J]. Chem Eng J,2018,349:438−455. [28] DUBININ M M. The potential theory of adsorption of gases and vapors for adsorption with energetically nonuniform surface[J]. Chem Rev, 1960, 60(2): 235–241. [29] BENARD P, CHAHINE R. Modeling of high-pressure adsorption isotherms above the critical temperature on microporous adsorbents: Application to methane[J]. Langmuir,1997,13(4):808−813. doi: 10.1021/la960843x [30] 郑青榕, 顾安忠, 杨晓东, 林文胜, 鲁雪生. 氢气在炭狭缝孔内吸附的预测[J]. 化学物理学报,2001,14(6):675−680.ZHENG Qing-rong, GU An-zhong, YANG Xiao-dong, LIN Wen-sheng, LU Xue-sheng. Prediction of hydrogen adsorption in carbon slit pores[J]. Chin J Chem Phys,2001,14(6):675−680. [31] 郑青榕. 多壁碳纳米管吸附储氢研究[D]. 上海: 上海交通大学, 2002.ZHENG Qing-rong. A study of hydrogen storage by adsorption on multi-walled carbon nanotube[D]. Shanghai: Shanghai Jiao Tong University, 2002. -




 下载:
下载: