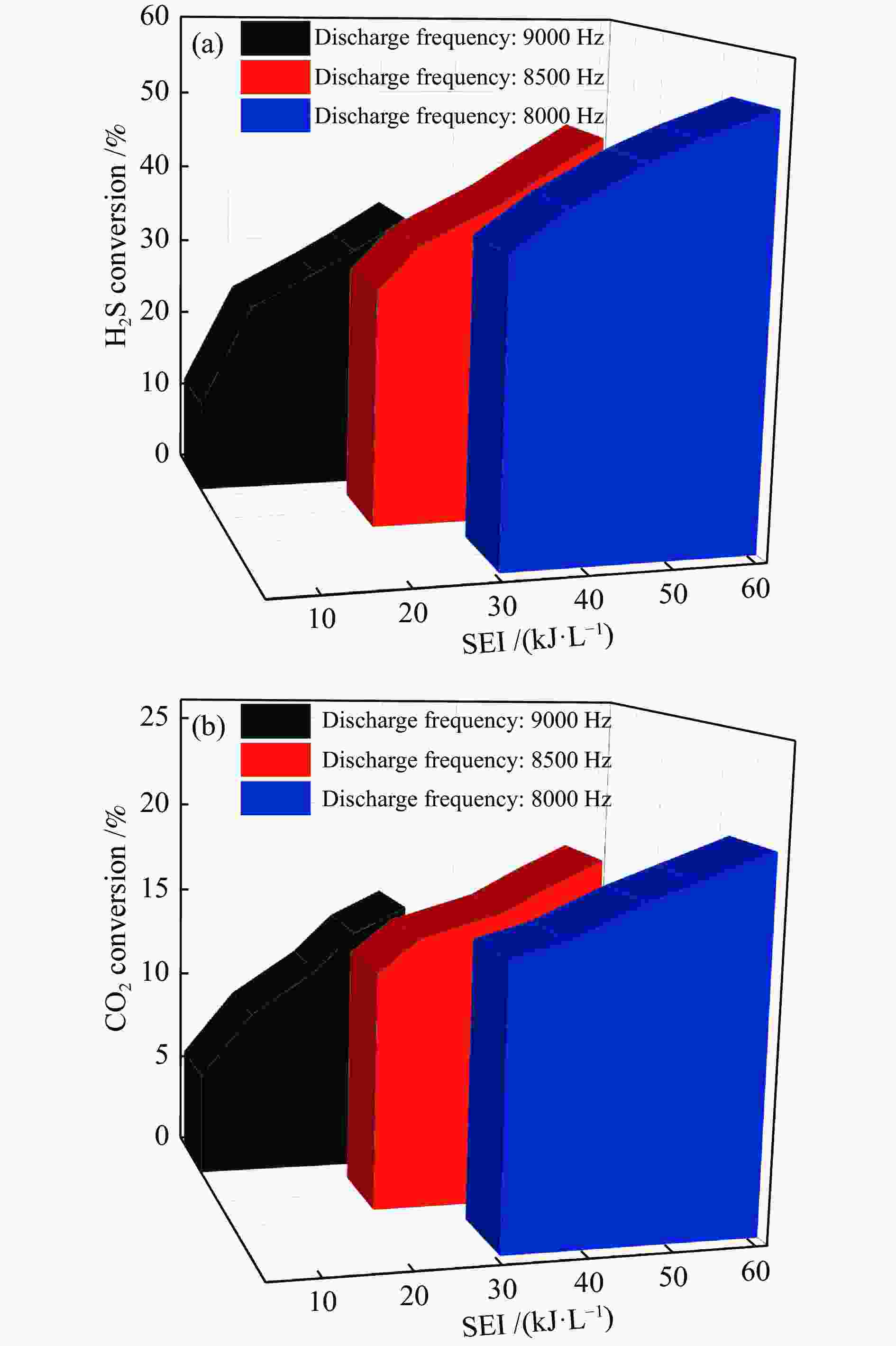
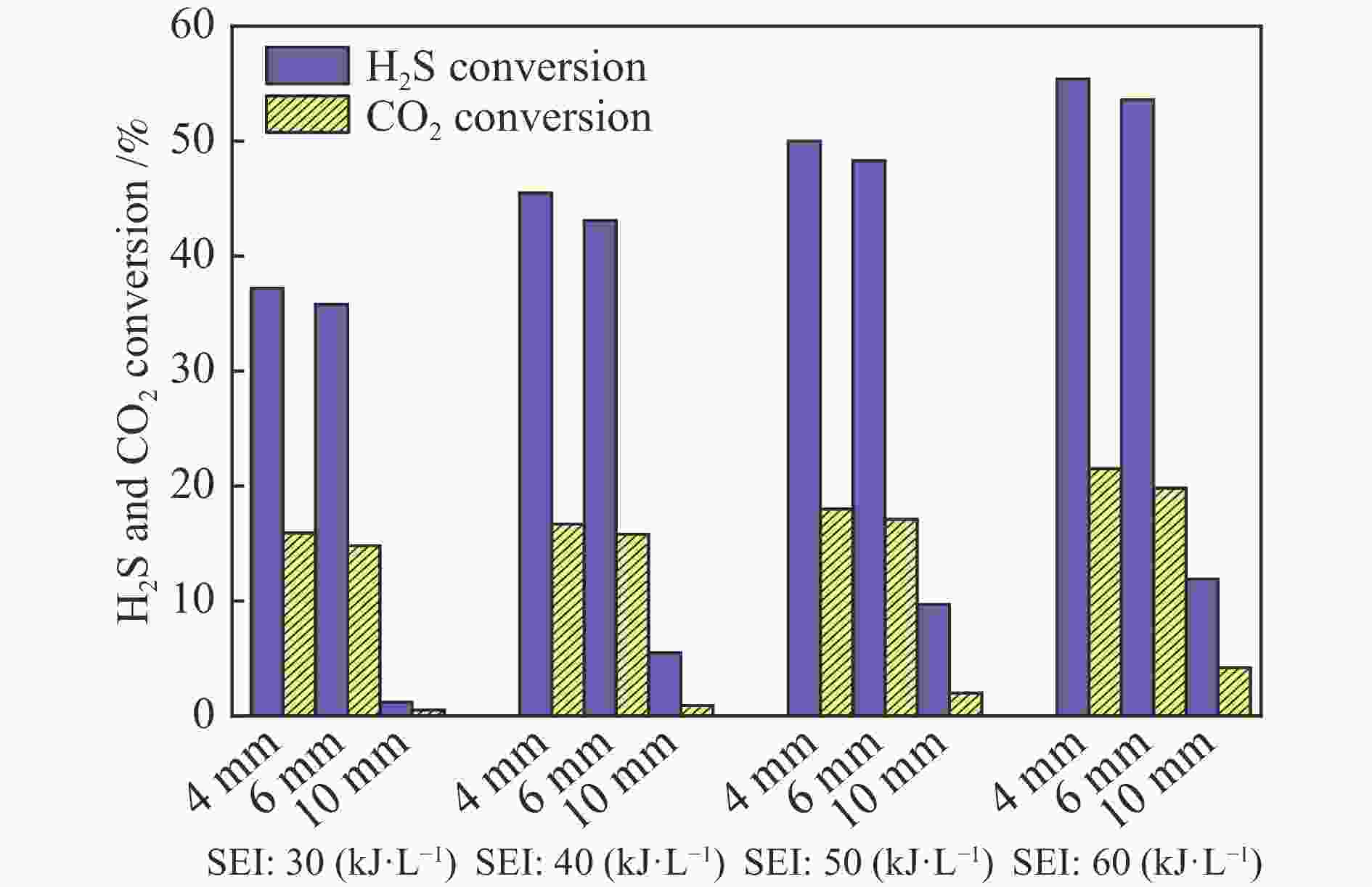
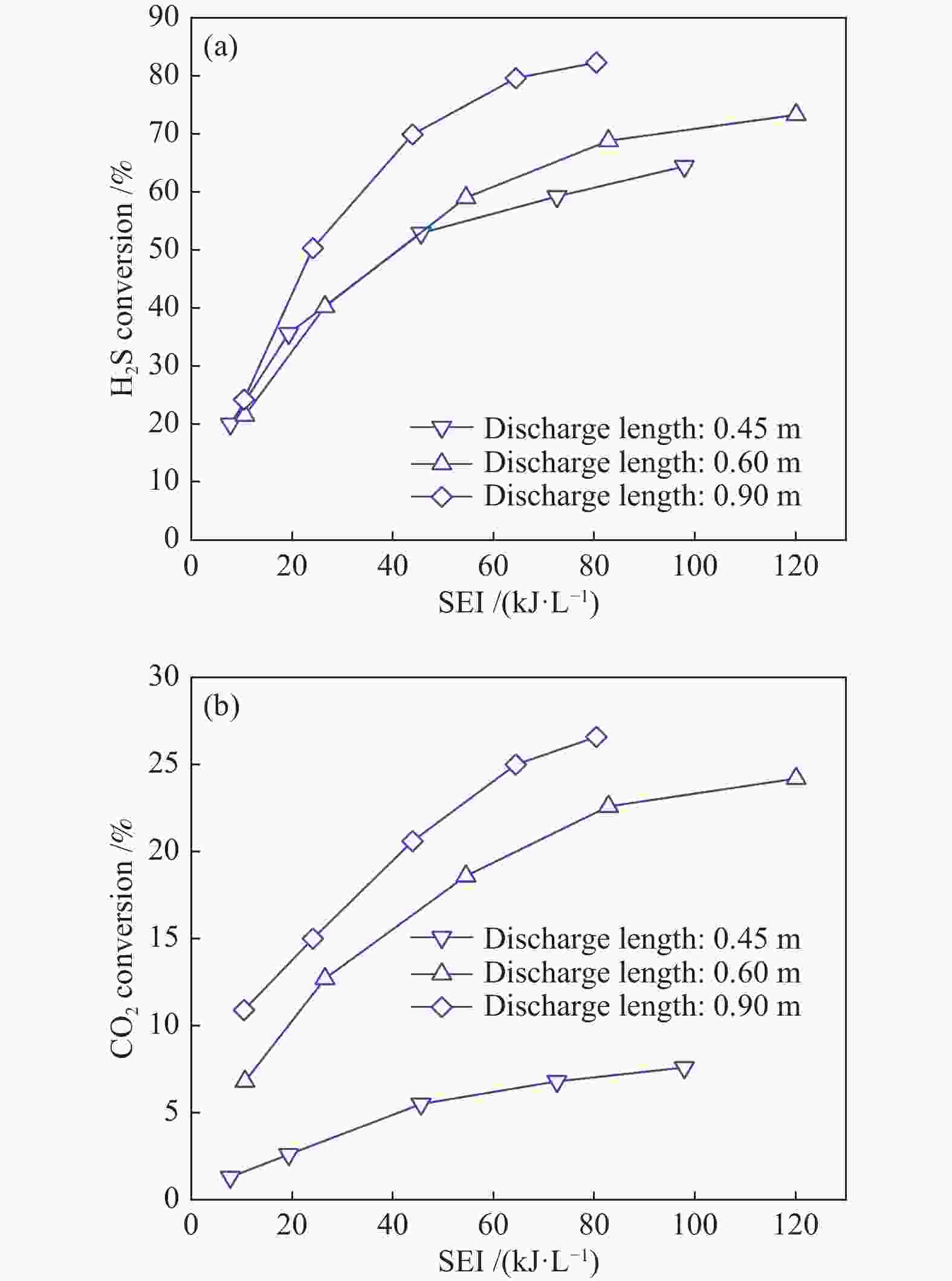
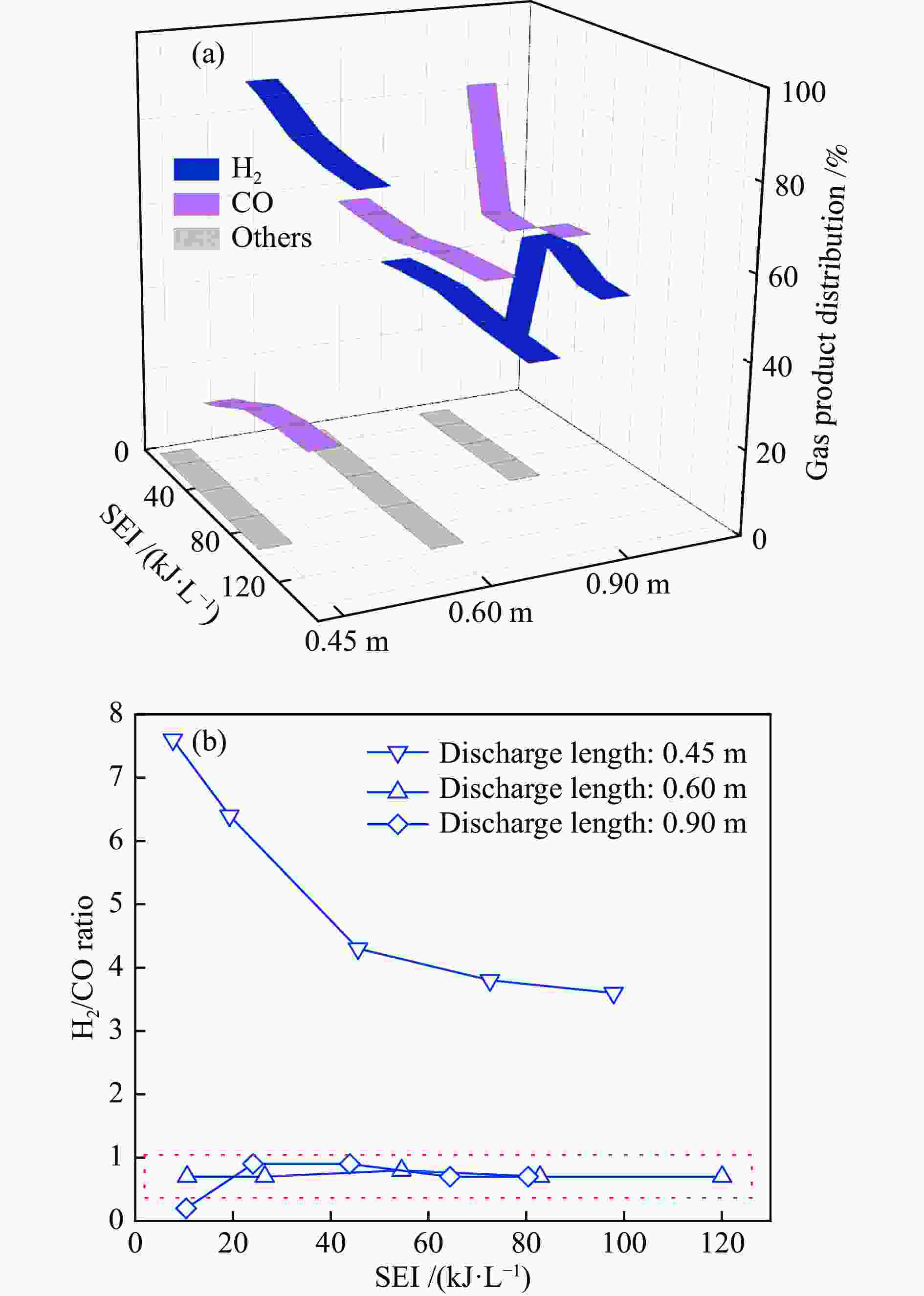
The influence factors of dielectric barrier discharge plasma to production of syngas derived from H2S-CO2 acid gas
-
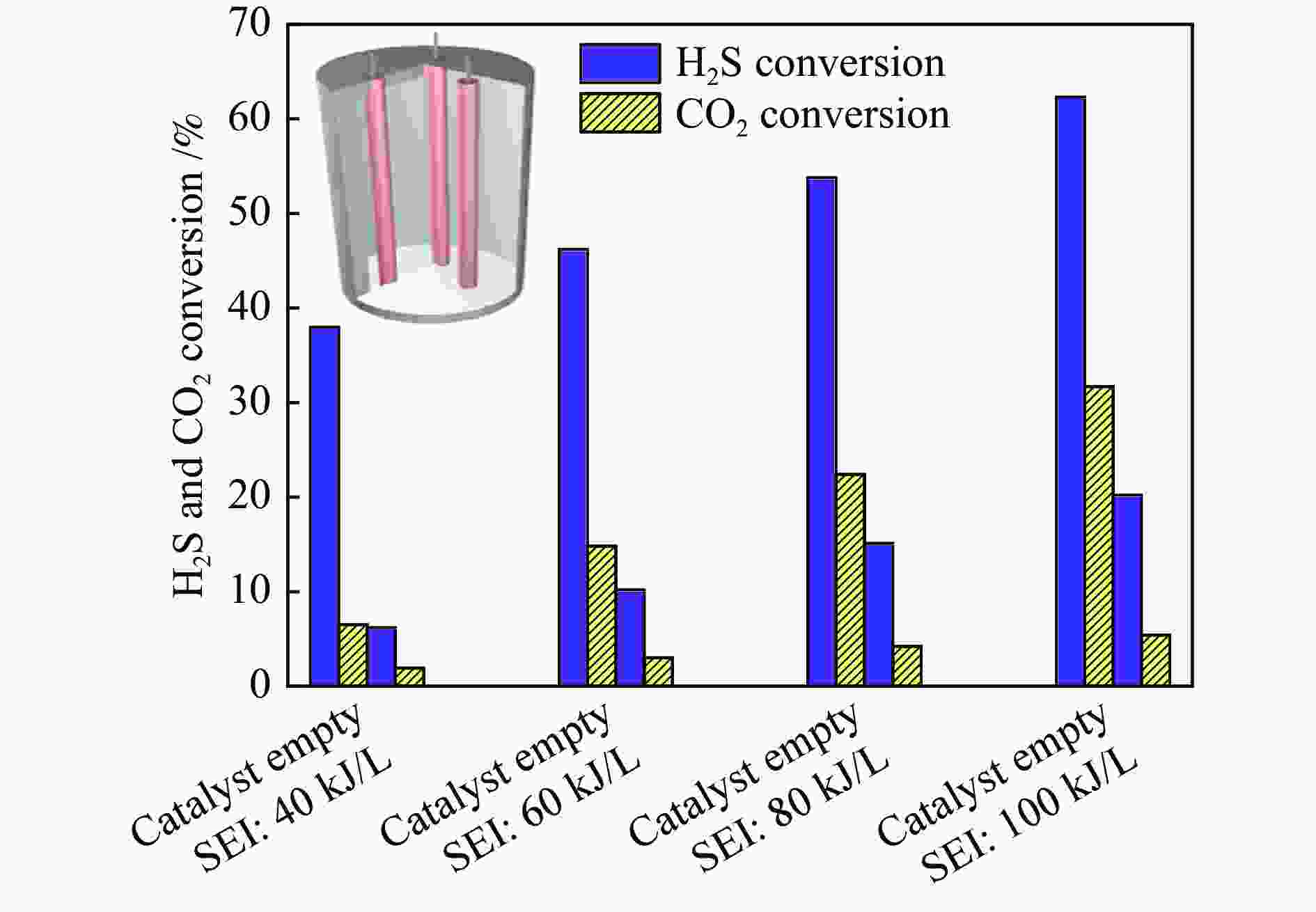
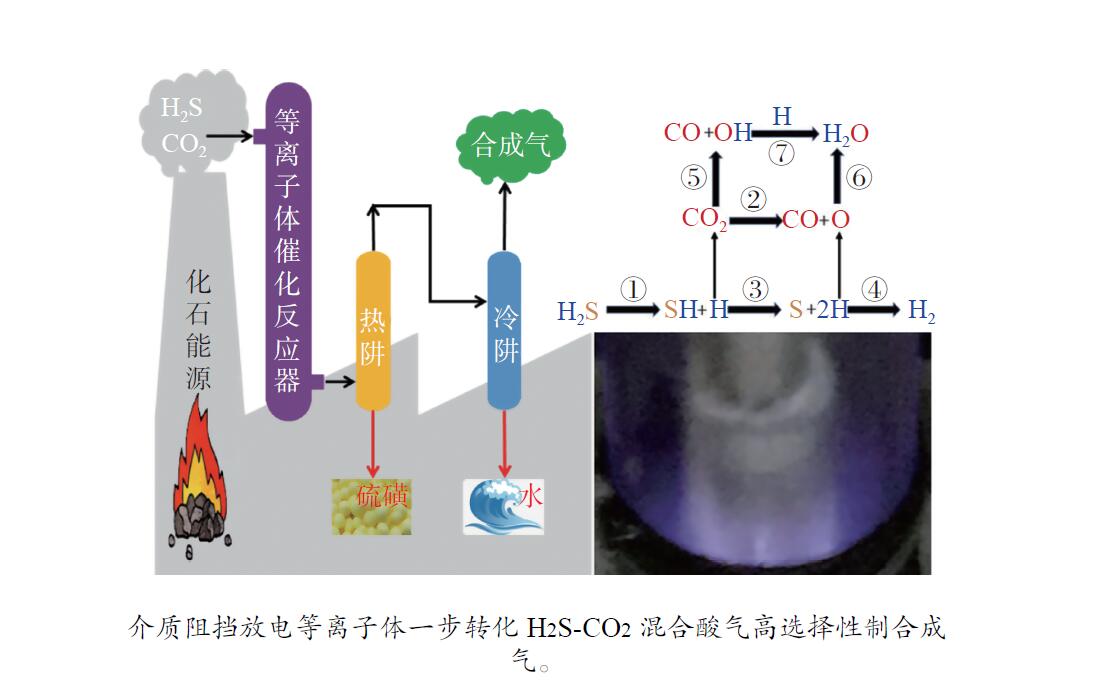
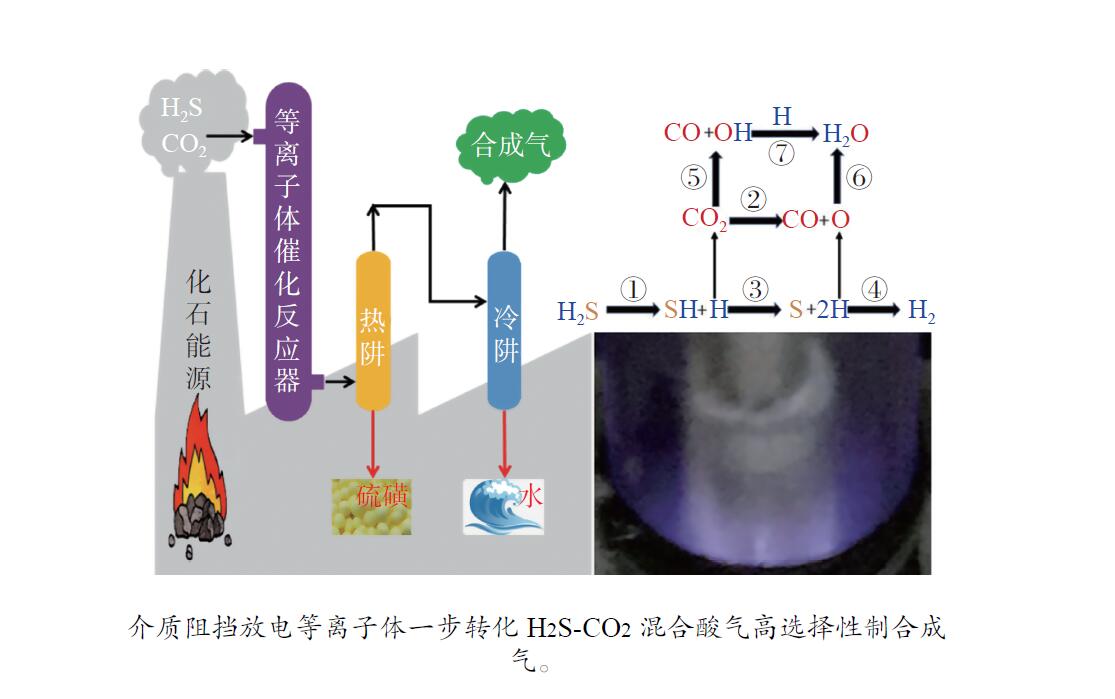
摘要: H2S和CO2两种有害酸性废气常共存于煤化工、天然气化工及石油化工等重要化工生产中,造成了工业设备及管线腐蚀,必须就地进行无害化处理。采用介质阻挡放电等离子体催化实现一步转化H2S-CO2混合酸气制合成气,将具有强腐蚀性、毒性的H2S和温室气体CO2无害化,又产出合成气,完成了以废治废的酸性废气资源化再利用。研究了用于H2S-CO2一步转化制合成气的介质阻挡放电等离子体反应各参数对转化反应的影响,进行了不同参数的对比研究,考察并揭示了比能量密度(SEI)、放电结构、放电频率、放电间隙以及放电区域长度等与H2S-CO2转化制合成气反应性能的内在关联。在此基础上设计并构建了多管并联介质阻挡放电等离子体反应系统。Abstract: H2S and CO2, two harmful acid waste gases, often co-exist in important chemical production such as coal-chemical industry, natural gas chemical industry and petrochemical industry, causing corrosion of industrial equipment and pipelines, and must be treated innocuously. Co-conversion of H2S-CO2 mixed acid gas to syngas has been carried out using dielectric barrier discharge (DBD) plasma-catalysis, which renders the highly corrosive and toxic H2S and greenhouse gas CO2 harmless, and produces syngas. The effects of various parameters of the DBD plasma on the reaction of one-step conversion of H2S-CO2 to syngas were studied. Moreover, a comparative study of the different parameters of DBD plasma was carried out. The intrinsic correlation between the reaction performance of syngas production via H2S-CO2 conversion and these parameters, including specific energy input (SEI), discharge shape, discharge frequency, discharge gap and discharge length, was investigated and revealed. On this basis, a multi-tube parallel DBD plasma reaction system was designed and constructed.
-
Key words:
- syngas /
- hydrogen sulfide /
- carbon dioxide /
- non-thermal plasma /
- waste treatment
-
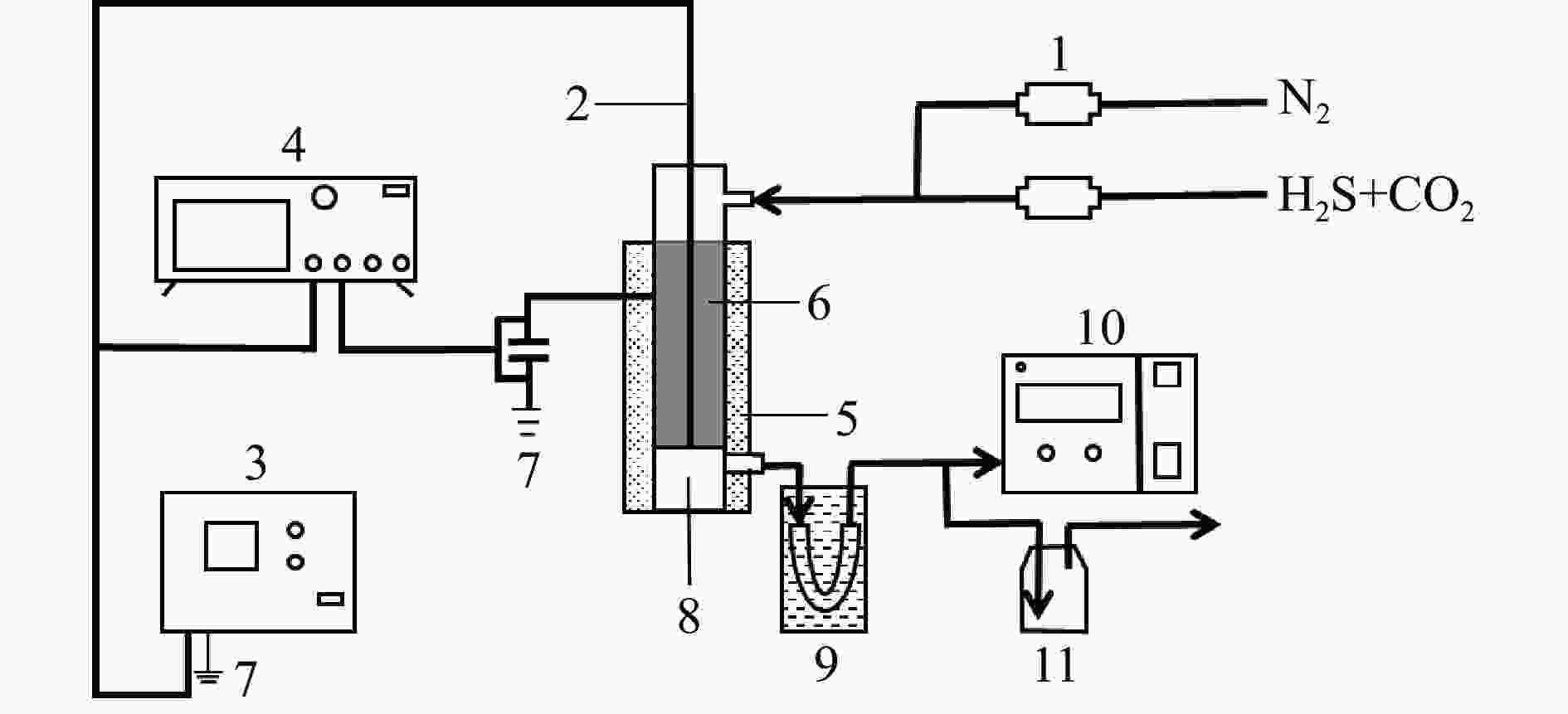
图 1 低温等离子体系统示意图
1:质量流量计;2:高压电极;3:等离子体发生器;4:数字示波器;5:油浴;6:等离子体反应器;7:接地极;8:积硫槽;9:冷阱;10:气相色谱分析仪;11:碱液处理
Figure 1 Schematic diagram of non-thermal plasma system
1: mass flow meter; 2: high-voltage electrode; 3: AC power supply; 4: digital oscilloscope; 5: oil bath; 6: non-thermal plasma reactor; 7: grounding electrode; 8: sulphur tank; 9: cold trap; 10: gas chromatograph; 11: lye treatment
-
[1] HENDRICKSON R G, CHANG A, HAMILTON R J. Co-worker fatalities from hydrogen sulfide[J]. Am J Ind Med,2004,45(4):346−350. doi: 10.1002/ajim.10355 [2] MA Y, GUO H, SELYANCHYN R, WANG B, DENG L, DAI Z, JIANG X. Hydrogen sulfide removal from natural gas using membrane technology: A review[J]. J Mater Chem A,2021,9:20211−20240. doi: 10.1039/D1TA04693D [3] FELLAH M F. Adsorption of hydrogen sulfide as initial step of H2S removal: A DFT study on metal exchanged ZSM-12 clusters[J]. Fuel Process Technol,2016,144:191−196. doi: 10.1016/j.fuproc.2016.01.003 [4] SASSI M, AMIRA N. Chemical reactor network modeling of a microwave plasma thermal decomposition of H2S into hydrogen and sulfur[J]. Int J Hydrog Energy,2012,37(13):10010−10019. doi: 10.1016/j.ijhydene.2012.04.006 [5] KHAIRULIN S, KERZHENTSEV M, SALNIKOV A, ISMAGILOV Z R. Direct selective oxidation of hydrogen sulfide: Laboratory, pilot and industrial tests[J]. Catalysts,2021,11(9):1109. doi: 10.3390/catal11091109 [6] YADAV S, MONDAL S S. A review on the progress and prospects of oxy-fuel carbon capture and sequestration (CCS) technology[J]. Fuel,2022,308:122057. doi: 10.1016/j.fuel.2021.122057 [7] D’ALESSANDRO D M, SMIT B, LONG J R. Carbon dioxide capture: Prospects for new materials[J]. Angew Chem Int Ed,2010,49(35):6058−6082. doi: 10.1002/anie.201000431 [8] JONES C W, MAGINN E J. Materials and processes for carbon capture and sequestration[J]. ChemSusChem,2010,3(8):863−864. doi: 10.1002/cssc.201000235 [9] LI Z, CAI J, GAO Y, ZHANG L, LIANG Q, HAO W, JIANG Y, ZENG J R. Efficient production of medium chain fatty acids in microbial electrosynthesis with simultaneous bio-utilization of carbon dioxide and ethanol[J]. Bioresour Technol,2022,352:127101. doi: 10.1016/j.biortech.2022.127101 [10] APPEL A M, BERCAW J E, BOCARSLY A B, DOBBEK H, DUBOIS D L, DUPUIS M, FERRY J G, FUJITA E, HILLE R, KENIS P J A, KERFELD C A, MORRIS R H, PEDEN C H F, PORTIS A R, RAGSDALE S W, RAUCHFUSS T H, REEK J N H, SEEFELDT L C, THAUER R K, WALDROPG L. Frontiers, opportunities, and challenges in biochemical and chemical catalysis of CO2 fixation[J]. Chem Rev,2013,113(8):6621−6658. doi: 10.1021/cr300463y [11] XU X, MARTIN G J O, KENTISH S E. Enhanced CO2 bio-utilization with a liquid-liquid membrane contactor in a bench-scale microalgae raceway pond[J]. J CO2 Util,2019,34:207−214. doi: 10.1016/j.jcou.2019.06.008 [12] 王晗, 樊升, 王森, 董梅, 秦张峰, 樊卫斌, 王建国. 二氧化碳加氢制一些烃类化合物的研究进展[J]. 燃料化学学报,2021,49(11):1609−1619. doi: 10.1016/S1872-5813(21)60122-6WANG Han, FAN Sheng, WANG Sen, DONG Mei, QIN Zhang-feng, FAN Wei-bin, WANG Jian-guo. Research progresses in the hydrogenation of carbon dioxide to certain hydrocarbon products[J]. J Fuel Chem Technol,2021,49(11):1609−1619. doi: 10.1016/S1872-5813(21)60122-6 [13] ARESTA M, DIBENEDETTO A, ANGELINI A. Catalysis for the valorization of exhaust carbon: From CO2 to chemicals, materials, and fuels. Technological use of CO2[J]. Chem Rev,2014,114(3):1709−1742. doi: 10.1021/cr4002758 [14] GUO Y, GUO X, SONG C, HAN X, LIU H, ZHAO Z. Capsule-structured copper-zinc catalyst for highly efficient hydrogenation of carbon dioxide to methanol[J]. ChemSusChem,2019,12(22):4916−4926. doi: 10.1002/cssc.201902485 [15] 刘昌俊, 郭秋婷, 叶静云, 孙楷航, 范志刚, 葛庆峰. 二氧化碳转化催化剂研究进展及相关问题思考[J]. 化工学报,2016,67(1):6−13.LIU Chang-jun, GUO Qiu-ting, YE Jing-yun, SUN Kai-hang, FAN Zhi-gang, GE Qing-feng. Perspective on catalyst investigation for CO2 conversion and related issues[J]. CIESC J,2016,67(1):6−13. [16] ZHOU W, CHENG K, KANG J, ZHOU C, SUBRAMANIAN V, ZHANG Q, WANG Y. New horizon in C1 chemistry: Breaking the selectivity limitation in transformation of syngas and hydrogenation of CO2 into hydrocarbon chemicals and fuels[J]. Chem Soc Rev,2019,48(12):3193−3228. doi: 10.1039/C8CS00502H [17] ZHANG F, WEI Z, JIANG G, LI G, ZHAO M, ZHANG Z, CHENG J, HAO Z. Synergistic conversion of acid gases (H2S and CO2) to valuable chemicals: Carbonyl sulfide synthesis over vacancy-defective CoMo sulfide catalysts[J]. Appl Catal B: Environ,2022,319:121912. doi: 10.1016/j.apcatb.2022.121912 [18] MA W, WANG H, YU W, WANG X, XU Z, ZONG X, LI C. Achieving simultaneous CO2 and H2S conversion via a coupled solar‐driven electrochemical approach on non‐precious‐metal catalysts[J]. Angew Chem Int Ed,2018,57(13):3473−3477. doi: 10.1002/anie.201713029 [19] 吴秀章. 煤制低碳烯烃工艺与工程[M]. 北京: 化学工业出版社, 2014.WU Xiu-zhang. Coal-to-olefins Technology and Engineering[M]. Beijing: Chemical Industry Press, 2014. [20] CRISCI A G D, MONIRI A, XU Y. Hydrogen from hydrogen sulfide: Towards a more sustainable hydrogen economy[J]. Int J Hydrogen Energy,2019,44(3):1299−1327. doi: 10.1016/j.ijhydene.2018.10.035 [21] 张文杰, 侯美伶, 周兴, 黄河, 岑望来. 基于第一性原理计算硫化氢(H2S)在PT-GRAPHENE上的吸附性能和解离机理[J]. 燃料化学学报,2022,50(9):1211−1220. doi: 10.1016/S1872-5813(22)60023-9ZHANG Wen-jie, HOU Mei-ling, ZHOU Xing, HUANG He, CEN Wang-lai. A theoretical study of H2S adsorption and dissociation mechanism on defected graphene doped with Pt[J]. J Fuel Chem Technol,2022,50(9):1211−1220. doi: 10.1016/S1872-5813(22)60023-9 [22] JEFFRY L, ONG M Y, NOMANBHAY S, MOFIJUR M, MUBASHIR M, SHOW P L. Greenhouse gases utilization: A review[J]. Fuel,2021,301:121017. doi: 10.1016/j.fuel.2021.121017 [23] ZHANG B, BAI J, ZHANG Y, ZHOU C, WANG P, ZHA L, LI J, SIMCHI A, ZHOU B. High yield of CO and synchronous S recovery from the conversion of CO2 and H2S in natural gas based on a novel electrochemical reactor[J]. Environ Sci Technol,2021,55(21):14854−14862. doi: 10.1021/acs.est.1c04414 [24] GROISIL M, IBRAHIM S, GUPTA A K, ALSHOAIBI A. Numerical examination of acid gas for syngas and sulfur recovery[J]. Energy Procedia,2015,75:3066−3070. doi: 10.1016/j.egypro.2015.07.628 [25] IBRAHIM S, RAJ A. Kinetic simulation of acid gas (H2S and CO2) destruction for simultaneous syngas and sulfur recovery[J]. Ind Eng Chem Res,2016,55(24):6743−6752. doi: 10.1021/acs.iecr.6b01176 [26] SU H, LI Y, LI P, CHEN Y, ZHANG Z, FANG X. Simultaneous recovery of carbon and sulfur resources from reduction of CO2 with H2S using catalysts[J]. J Energy Chem,2016,25(1):110−116. doi: 10.1016/j.jechem.2015.08.009 [27] WU P, LI X, ULLAH N, LI Z. Synergistic effect of catalyst and plasma on CO2 decomposition in a dielectric barrier discharge plasma reactor[J]. Mol Catal,2020,499:111304. [28] ELLMER K, LICHTENBERGER D. Plasma diagnostics by energy resolved quadrupole mass spectrometry of a reactive magnetron sputtering discharge from an Fe target in Ar-H2S atmospheres[J]. Surf Coat Technol,1995,74:586−593. [29] MICHIELSEN I, UYTDENHOUWEN Y, PYPE J, MICHIELSEN B, MERTENS J, RENIERS F, MEYNEN V, BOGAERTS A. CO2 dissociation in a packed bed DBD reactor: First steps towards a better understanding of plasma catalysis[J]. Chem Eng J,2017,326:477−488. doi: 10.1016/j.cej.2017.05.177 [30] CHEN G, SNYDERS R, BRITUN N. CO2 conversion using catalyst-free and catalyst-assisted plasma-processes: Recent progress and understanding[J]. J CO2 Util,2021,49:101557. doi: 10.1016/j.jcou.2021.101557 [31] ZHAO L, WANG Y, WANG A, LI X, SONG C, HU Y. Cr-doped ZnS semiconductor catalyst with high catalytic activity for hydrogen production from hydrogen sulfide in non-thermal plasma[J]. Catal Today,2019,337:83−89. doi: 10.1016/j.cattod.2019.02.032 [32] GEORGE A, SHEN B, CRAVEN M, WANG Y, KANG D. A review of non-thermal plasma technology: A novel solution for CO2 conversion and utilization[J]. Renewable Sustainable Energy Rev,2021,135:109702. doi: 10.1016/j.rser.2020.109702 [33] ZHAO L, LIU X, MU X, LI Y, FANG K. Highly selective conversion of H2S-CO2 to syngas by combination of non-thermal plasma and MoS2/Al2O3[J]. J CO2 Util,2020,37:45−54. doi: 10.1016/j.jcou.2019.11.021 [34] LIU X, ZHAO L, LI Y, FANG K, WU M. Ni-Mo sulfide semiconductor catalyst with high catalytic activity for one-step conversion of CO2 and H2S to syngas in non-thermal plasma[J]. Catalysts,2019,9(6):525. doi: 10.3390/catal9060525 [35] 王乾浩, 赵璐, 孙付琳, 房克功. ZSM-5 催化剂与低温等离子体协同转化H2S-CO2制合成气[J]. 化工学报,2022,73(1):255−265.WANG Qian-hao, ZHAO Lu, SUN Fu-lin, FANG Ke-gong. Production of syngas derived from H2S-CO2 via synergy of ZSM-5 catalyst and non-thermal plasma[J]. CIESC J,2022,73(1):255−265. [36] 房克功, 赵璐, 李文斌, 张立功, 周娟, 穆晓亮. 转化二氧化碳和硫化氢混合气制取合成气的方法及装置: 中国, 107244652B[P]. 2019-12-06.FANG Ke-gong, ZHAO Lu, LI Wen-bin, ZHANG Li-gong, ZHOU Juan, MU Xiao-liang. Method and apparatus for converting carbon dioxide and hydrogen sulfide mixture to produce syngas: CN, 107244652B[P]. 2019-12-06. [37] 骆嘉钦, 刘露, 马晓迅. 低温等离子体结合乙醇胺船舶尾气脱硫脱硝研究[J]. 燃料化学学报,2021,49(4):564−572.LUO Jia-qin, LIU Lu, MA Xiao-xun. Desulfurization and denitrification of the marine diesel exhaust by non-thermal plasma method with the addition of monoethanolamine[J]. J Fuel Chem Technol,2021,49(4):564−572. [38] 李莹, 赵璐, 刘晓展, 曾春新, 房克功. 低温等离子体制备低碳醇合成用KNiMo基催化剂及其结构性能表征[J]. 燃料化学学报,2019,47(5):513−522. doi: 10.1016/S1872-5813(19)30023-4LI Ying, ZHAO Lu, LIU Xiao-zhan, ZENG Chun-xin, FANG Ke-gong. Preparation of KNiMo-based catalysts by using non-thermal plasma and their catalytic performance in the synthesis of higher alcohols from syngas[J]. J Fuel Chem Technol,2019,47(5):513−522. doi: 10.1016/S1872-5813(19)30023-4 [39] KIM H H, LEE Y H, OGATA A, FUTAMURA S. Plasma-driven catalyst processing packed with photocatalyst for gas-phase benzene decomposition[J]. Catal Commun,2003,4(7):347−351. doi: 10.1016/S1566-7367(03)00086-4 [40] MEI D, ZHU X, HE Y L, YAN J D, TU X. Plasma-assisted conversion of CO2 in a dielectric barrier discharge reactor: Understanding the effect of packing materials[J]. Plasma Sources Sci Technol,2014,24(1):015011. doi: 10.1088/0963-0252/24/1/015011 [41] 于欣, 党小庆, 李世杰, 黄准, 郭惠, 张金龙. 单介质和双介质阻挡放电低温等离子体降解甲苯的比较[J]. 环境工程学报,2020,14(4):1033−1041. doi: 10.12030/j.cjee.201906002YU Xin, DANG Xiao-qing, LI Shi-jie, HUANG Zhun, GUO Hui, ZHANG Jin-long. Comparison of single and double dielectric barrier discharge non-thermal plasma for toluene removal[J]. Chin J Environ Eng,2020,14(4):1033−1041. doi: 10.12030/j.cjee.201906002 [42] TANG X, GAO F, WANG J, YI H, ZHAO S, ZHANG Y, WANG Z. Comparative study between single-and double-dielectric barrier discharge reactor for nitric oxide removal[J]. Ind Eng Chem Res,2014,53(14):6197−6203. doi: 10.1021/ie403932c [43] 邵涛, 严萍. 大气压气体放电及其等离子体应用[M]. 北京: 科学出版社, 2015.SHAO Tao, YAN Ping. Atmospheric Gas Discharge and its Plasma Application[M]. Beijing: Science Press, 2015. [44] 陈波. 大气压脉冲介质阻挡放电特性及放电参数效应研究[D]. 济南: 山东大学, 2013.CHEN Bo. Study oil characteristics of atmospheric-pressure pulsed dielectric barrier discharge and parameter effects of discharge[D]. Jinan: Shandong University, 2013. [45] LU N, ZHANG C, SHANG K, JIANG N, LI J, WU Y. Dielectric barrier discharge plasma assisted CO2 conversion: Understanding the effects of reactor design and operating parameters[J]. J Phys D: Appl Phys,2019,52(22):224003. doi: 10.1088/1361-6463/ab0ebb [46] REDDY E L, BIJU V M, SUBRAHMANYAM C. Production of hydrogen from hydrogen sulfide assisted by dielectric barrier discharge[J]. Int J Hydrog Energy,2012,37(3):2204−2209. doi: 10.1016/j.ijhydene.2011.10.118 [47] AERTS R, SOMERS W, BOGAERTS A. Carbon dioxide splitting in a dielectric barrier discharge plasma: A combined experimental and computational study[J]. ChemSusChem,2015,8(4):702−716. doi: 10.1002/cssc.201402818 [48] 李春茂, 董磊, 彭开晟, 魏文赋, 高国强, 吴广宁. 电极间隙对介质阻挡放电特性的影响[J]. 西南交通大学学报,2019,54(4):679−685.LI Chun-mao, DONG Lei, PENG Kai-sheng, WEI Wen-fu, GAO Guo-qiang, WU Guang-ning. Influence of electrode gap on characteristics of dielectric barrier discharge[J]. J Southwest Jiaotong Univ,2019,54(4):679−685. [49] 梁文俊, 李晶欣, 竹涛. 低温等离子体大气污染控制技术及应用[M]. 北京: 化学工业出版社, 2016.LIANG Wen-jun, LI Jing-xin, ZHU Tao. Air Pollution Control Technology and Application of Non-thermal Plasma[M]. Beijing: Chemical Industry Press, 2016. [50] REDDY E L, BIJU V M, SUBRAHMANYAM C. Production of hydrogen and sulfur from hydrogen sulfide assisted by nonthermal plasma[J]. Appl Energy,2012,95:87−92. doi: 10.1016/j.apenergy.2012.02.010 [51] MEI D, TU X. Conversion of CO2 in a cylindrical dielectric barrier discharge reactor: Effects of plasma processing parameters and reactor design[J]. J CO2 Util,2017,19:68−78. doi: 10.1016/j.jcou.2017.02.015 -




 下载:
下载: