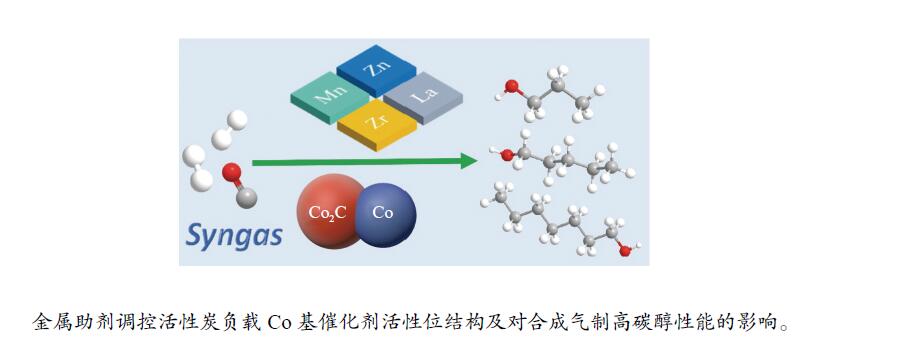
Effect of metal promoters on catalytic performance of Co/AC for higher alcohols synthesis from syngas
-
摘要: 本实验考察了不同金属助剂(Mn、Zn、La和Zr)对活性炭负载的Co基催化剂(Co/AC)在合成气转化中的活性和产物选择性调控的影响。结果表明,这些金属助剂对CO解离速率,Co2C生成以及醇类产物选择性都有明显的促进作用。所构筑的Co2C/Co0 构成了高碳醇合成所需的双活性位结构。其中,Zn修饰的Co/AC 催化剂表现出最强的CO解离速率、最高的活性和醇类产物的时空收率。Mn助剂最有利于Co2C的生成,但过高的Co2C/Co0比例导致活性略有下降。Zr和La助剂修饰的催化剂具有相似的CO解离速率和催化活性,但CoZr/AC催化剂适宜的Co2C/Co0比及界面环境更有利于实现CO解离和CO非解离功能的协同,从而表现出最高的醇产物选择性。Abstract: Shifting products of Fischer-Tropsch Synthesis (FTS) from paraffins to value-added higher alcohols receives great attention but remains great challenge. Herein, metal oxides of Mn, Zn, La and Zr are investigated as promoters to tune the activity and product distributions of Co/AC catalyst for syngas conversion. It is found that these promoters show different promotion effect on CO dissociation rate, the formation of Co2C phase and the alcohols selectivity. The formed Co2C/Co0 constitutes the dual active site for higher alcohols synthesis. The strongest CO dissociation rate is observed for Zn-promoted Co/AC catalyst, resulting in the highest activity and space-time yield (STY) of alcohols. The Mn promoter is most conducive to the formation of Co2C, but slightly decreases the activity. The similar CO dissociation rate and CO conversion are obtained over both Zr- and La-promoted Co/AC catalysts, but the Zr-promoted Co/AC catalyst exhibits the highest alcohols selectivity due to the function balance between CO non-dissociative insertion and CO dissociation.
-
Key words:
- syngas /
- higher alcohols /
- CO hydrogenation /
- promoter /
- cobalt carbide
-
Table 1 Textural properties of the fresh CoX/AC catalysts
Catalyst SBET /
(m2·g−1)Pore size /
nmPore volume /
(cm3·g−1, STP)AC 1396.0 2.1 0.66 Co/AC 319.1 2.1 0.11 CoMn/AC 416.2 2.1 0.13 CoZn/AC 368.1 2.1 0.12 CoLa/AC 409.5 2.1 0.12 CoZr/AC 492.6 2.1 0.11 Table 2 Average nanoparticle sizes of Co species over spent CoX/AC catalysts characterized by HRTEM and XRD
Catalyst d /nm TEMa XRD (Co2C/Co) Co/AC 11.2 23.0/12.0 CoMn/AC 11.3 16.3/− CoZn/AC 20.6 21.7/15.5 CoLa/AC 12.3 19.4/10.6 CoZr/AC 12.1 16.9/11.3 a: Co-containing species Table 3 Relative content of cobalt species in the spent CoX/AC catalysts
Catalyst Co2C Co0 Co2C/ Co0 Co/AC 23.4 76.6 0.31 CoMn/AC 93.9 6.1 15.39 CoZn/AC 50.1 49.9 1.00 CoLa/AC 71.7 28.3 2.53 CoZr/AC 63.6 36.4 1.75 Table 4 Catalytic performance of various catalysts for syngas conversiona
Catalyst CO conversion /% Selectivity /C% STYb /(mg·g−1·h−1) ROHc R=d Re CH4 CO2 ROH + R= ROH R= R Co/AC 18.0 12.1 22.1 64.6 30.7 1.2 34.1 15.8 19.8 82.4 CoZn/AC 29.7 13.9 12.5 72.6 24.9 1.0 26.4 29.2 18.7 133.4 CoLa/AC 18.4 14.4 29.8 54.4 23.9 1.4 44.2 17.3 24.8 73.8 CoMn/AC 15.4 17.5 36.5 45.0 13.0 1.0 54.0 18.1 17.8 61.6 CoZr/AC 19.0 19.3 21.7 58.0 25.0 1.0 41.0 24.7 21.0 81.3 a: Reaction conditions: H2/CO=2, 4 MPa, 220 °C, 3000 mL/(g∙h); b: space-time yield; c: oxygenates including alcohols and aldehydes;
d: olefins; e: paraffinsTable 5 Catalyst surface carbon species concentration
Catalyst Integrated peak area (*E−5) Co/AC 1.10 CoZr/AC 1.30 CoLa/AC 1.36 CoMn/AC 1.43 CoZn/AC 4.02 -
[1] CHENG K, KANG J C, KING D L, SUBRAMANIAN V, ZHOU C, ZHANG Q H, WANG Y. Advances in catalysis for syngas conversion to hydrocarbons[J]. Adv Catal,2017,60:125−208. [2] KHODAKOV A Y, CHU W, FONGARLAND P. Advances in the development of novel cobalt Fischer-Tropsch catalysts for synthesis of long-chain hydrocarbons and clean fuels[J]. Chem Rev,2007,107(5):1692−1744. doi: 10.1021/cr050972v [3] LIN T J, YU F, AN Y L, QIN T T, LI L S, GONG K, ZHONG L S, SUN Y H. Cobalt carbide nanocatalysts for efficient syngas conversion to value-added chemicals with high selectivity[J]. Acc Chem Res,2021,54(8):1961−1971. doi: 10.1021/acs.accounts.0c00883 [4] LIN T J, AN Y L, YU F, GONG K, YU H L, WANG C Q, SUN Y H, ZHONG L S. Advances in selectivity control for Fischer-Tropsch synthesis to fuels and chemicals with high carbon efficiency[J]. ACS Catal,2022,12(19):12092−12112. doi: 10.1021/acscatal.2c03404 [5] TORRES GALVIS H M, BITTER J H, KHARE C B, RUITENBEEK M, DUGULAN A I, DE JONG K P. Supported iron nanoparticles as catalysts for sustainable production of lower olefins[J]. Science,2012,335(6070):835−838. doi: 10.1126/science.1215614 [6] ZHONG L S, YU F, AN Y L, ZHAO Y H, SUN Y H, LI Z J, LIN T J, LIN Y J, QI X Z, DAI Y Y, GU L, HU J S, JIN S F, SHEN Q, WANG H. Cobalt carbide nanoprisms for direct production of lower olefins from syngas[J]. Nature,2016,538(7623):84−87. doi: 10.1038/nature19786 [7] LIN T J, QI X Z, WANG X X, XIA L, WANG C Q, YU F, WANG H, LI S G, ZHONG L S, SUN Y H. Direct production of higher oxygenates by syngas conversion over a multifunctional catalyst[J]. Angew Chem Int Ed,2019,58(14):4627−4631. doi: 10.1002/anie.201814611 [8] XU Y F, LI X Y, GAO J H, WANG J, MA G Y, WEN X D, YANG Y, LI Y W, DING M Y. A hydrophobic FeMn@Si catalyst increases olefins from syngas by suppressing C1 by-products[J]. Science,2021,371(6529):610−613. doi: 10.1126/science.abb3649 [9] ZHAO B, ZHAI P, WANG P F, LI J Q, LI T, PENG M, ZHAO M, HU G, YANG Y, LI Y W, ZHANG Q W, FAN W B, MA D. Direct transformation of syngas to aromatics over Na-Zn-Fe5C2 and hierarchical HZSM-5 tandem catalysts[J]. Chem,2017,3(2):323−333. doi: 10.1016/j.chempr.2017.06.017 [10] LUK H T, MONDELLI C, FERRÉ D C, STEWART J A, PÉREZ-RAMÍREZ J. Status and prospects in higher alcohols synthesis from syngas[J]. Chem Soc Rev,2017,46(5):1358−1426. doi: 10.1039/C6CS00324A [11] AN Y L, LIN T J, YU F, YANG Y Z, ZHONG L S, WU M H, SUN Y H. Advances in direct production of value-added chemicals via syngas conversion[J]. Sci China Chem,2017,60(7):887−903. doi: 10.1007/s11426-016-0464-1 [12] BEIRAMAR J M, GRIBOVAL-CONSTANT A, KHODAKOV A Y. Effects of metal promotion on the performance of CuZnAl catalysts for alcohol synthesis[J]. ChemCatChem,2014,6(6):1788−1793. doi: 10.1002/cctc.201402037 [13] PEI Y P, LIU J X, ZHAO Y H, DING Y J, LIU T, DONG W D, ZHU H J, SU H Y, YAN L, LI J L, LI W X. High alcohols synthesis via Fischer-Tropsch reaction at cobalt metal/carbide interface[J]. ACS Catal,2015,5(6):3620−3624. doi: 10.1021/acscatal.5b00791 [14] XIANG Y, KRUSE N. Tuning the catalytic CO hydrogenation to straight- and long-chain aldehydes/alcohols and olefins/paraffins[J]. Nat Commun,2016,7(1):13058. doi: 10.1038/ncomms13058 [15] YANG Y Z, LIN T J, QI X Z, YU F, AN Y L, LI Z J, DAI Y Y, ZHONG L S, WANG H, SUN Y H. Direct synthesis of long-chain alcohols from syngas over CoMn catalysts[J]. Appl Catal A: Gen,2018,549:179−187. doi: 10.1016/j.apcata.2017.09.037 [16] AN Y L, LIN T J, YU F, WANG X X, LU Y W, ZHONG L S, WANG H, SUN Y H. Effect of reaction pressures on structure-performance of Co2C-based catalyst for syngas conversion[J]. Ind Eng Chem Res,2018,57(46):15647−15653. doi: 10.1021/acs.iecr.8b03504 [17] ZHAO Z A, LU W, YANG R O, ZHU H J, DONG W D, SUN F F, JIANG Z, LYU Y, LIU T, DU H, DING Y J. Insight into the formation of Co@Co2C Catalysts for direct synthesis of higher alcohols and olefins from syngas[J]. ACS Catal,2018,8(1):228−241. doi: 10.1021/acscatal.7b02403 [18] ZHAO Z A, LU W, ZHU H J, DONG W D, LYU Y, LIU T, CHEN X K, WANG Y Q, DING Y J. Tuning the Fischer-Tropsch reaction over CoxMnyLa/AC catalysts toward alcohols: Effects of La promotion[J]. J Catal,2018,361:156−167. doi: 10.1016/j.jcat.2018.02.008 [19] LI Z J, ZHONG L S, YU F, AN Y L, DAI Y Y, YANG Y Z, LIN T J, LI S G, WANG H, GAO P, SUN Y H, HE M Y. Effects of sodium on the catalytic performance of CoMn catalysts for Fischer-Tropsch to olefin reactions[J]. ACS Catal,2017,7(5):3622−3631. doi: 10.1021/acscatal.6b03478 [20] LI Z J, YAO N, CEN J, LI X N, ZHONG L S, SUN Y H, HE M Y. Effects of alkali metal promoters on the structure-performance relationship of CoMn catalysts for Fischer-Tropsch synthesis[J]. Catal Sci Technol,2020,10(6):1816−1826. doi: 10.1039/C9CY02441G [21] LEBARBIER V M, MEI D, KIM D H, ANDERSEN A, MALE J L, HOLLADAY J E, ROUSSEAU R, WANG Y. Effects of La2O3 on the mixed higher alcohols synthesis from syngas over Co catalysts: A combined theoretical and experimental study[J]. J Phys Chem C,2011,115(35):17440−17451. doi: 10.1021/jp204003q [22] SINGH J A, HOFFMAN A S, SCHUMANN J, BOUBNOV A, ASUNDI A S, NATHAN S S, NØRSKOV J, BARE S R, BENT S F. Role of Co2C in ZnO-promoted Co catalysts for alcohol synthesis from syngas[J]. ChemCatChem,2019,11(2):799−809. doi: 10.1002/cctc.201801724 [23] QI X Z, LIN T J, GONG K, WANG X X, LV D, YU F, AN Y L, TANG Z Y, ZHONG L S. Direct synthesis of higher oxygenates via syngas over zinc oxide modified CoMn-based catalysts[J]. Appl Catal A: Gen,2022,648:118925. doi: 10.1016/j.apcata.2022.118925 [24] DU H, ZHU H J, CHEN X K, DONG W D, LU W, LUO W T, JIANG M, LIU T, DING Y J. Study on CaO-promoted Co/AC catalysts for synthesis of higher alcohols from syngas[J]. Fuel,2016,182:42−49. doi: 10.1016/j.fuel.2016.05.089 [25] LI L S, LIN T J, LI X, WANG C Q, QIN T T, AN Y L, LU Y W, ZHONG L S, SUN Y H. Control of Co0/Co2C dual active sites for higher alcohols synthesis from syngas[J]. Appl Catal A: Gen,2020,602:117704. doi: 10.1016/j.apcata.2020.117704 [26] TUCKER C L, RAGOO Y, MATHE S, MACHELI L, BORDOLOI A, ROCHA T C R, GOVENDER S, KOOYMAN P J, VAN STEEN E. Manganese promotion of a cobalt Fischer-Tropsch catalyst to improve operation at high conversion[J]. J Catal,2022,411:97−108. doi: 10.1016/j.jcat.2022.05.006 [27] JOHNSON G R, WERNER S, BELL A T. An investigation into the effects of Mn promotion on the activity and selectivity of Co/SiO2 for Fischer-Tropsch synthesis: Evidence for enhanced CO adsorption and dissociation[J]. ACS Catal,2015,5(10):5888−5903. doi: 10.1021/acscatal.5b01578 [28] LI Z J, LIN T J, YU F, AN Y L, DAI Y Y, LI S G, ZHONG L S, WANG H, GAO P, SUN Y H, HE M Y. Mechanism of the Mn promoter via CoMn spinel for morphology control: Formation of Co2C nanoprisms for Fischer-Tropsch to olefins reaction[J]. ACS Catal,2017,7(12):8023−8032. doi: 10.1021/acscatal.7b02144 [29] PEDERSEN E Ø, SVENUM I-H, BLEKKAN E A. Mn promoted Co catalysts for Fischer-Tropsch production of light olefins - An experimental and theoretical study[J]. J Catal,2018,361:23−32. doi: 10.1016/j.jcat.2018.02.011 [30] QIN T T, LIN T J, QI X Z, WANG C Q, LI L S, TANG Z Y, ZHONG L S, SUN Y H. Tuning chemical environment and synergistic relay reaction to promote higher alcohols synthesis via syngas conversion[J]. Appl Catal B: Environ,2021,285:119840. doi: 10.1016/j.apcatb.2020.119840 -





 下载:
下载: