Effect of molybdenum valence in low Mo/Sn ratio catalysts for the oxidation of methanol to dimethoxymethane
-
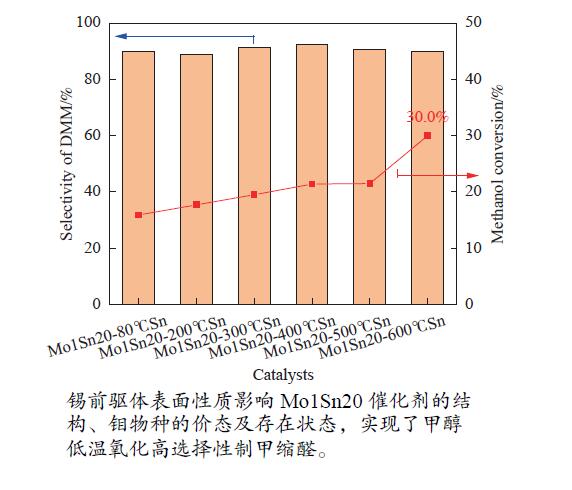
摘要: 采用两步水热合成法制备了一系列低Mo/Sn比(1∶20,物质的量比)催化剂,并考察了锡前驱体焙烧温度对甲醇氧化制甲缩醛反应性能的影响。通过XRD、Raman、FT-IR、XPS、NH3-TPD及H2-TPR等表征手段对催化剂的晶体结构、表面性质、氧化还原性及钼物种价态等进行了分析。结果表明,在Mo1Sn20-600℃Sn催化剂上,反应温度为140 ℃时,甲醇转化率及甲缩醛选择性分别达30.0%及90.0%。锡前驱体焙烧温度的变化主要影响了Mo1Sn20催化剂的结构、钼物种的价态及存在状态,进而影响其催化活性;高温焙烧的锡前驱体更有利于Mo1Sn20催化剂中甲醇活化的活性位点Mo6+物种的生成。
-
关键词:
- 甲醇 /
- 低温氧化 /
- 甲缩醛 /
- Mo1Sn20催化剂 /
- Mo6+ 物种
Abstract: A series of Mo/Sn (1:20, molar ratio) catalysts were prepared by two-step hydrothermal synthesis method, and the effect of calcination temperature of tin precursors on the reaction performance of methanol oxidation to dimethoxymethane (DMM) was investigated. The crystal structure, surface properties, redox property and valence change of molybdenum species of the catalyst were characterized by XRD, Raman, FT-IR, XPS, NH3-TPD and H2-TPR. The results showed that Mo1Sn20-600℃Sn catalyst exhibited better performance than other catalysts, achieving DMM selectivity of 90% with methanol conversion of 30% at 140 ℃. From the characterization results, the surface properties of the tin precursors affected the structure of catalyst, the degree of molybdenum oxide dispersion and valence of molybdenum species, and further influenced the performance of the catalysts. The high temperature calcination of tin precursors is more favorable for the generation of Mo6+ in the Mo1Sn20 catalyst.-
Key words:
- methanol /
- low temperature oxidation /
- dimethoxymethane /
- Mo1Sn20 catalyst /
- Mo6+ species
-
表 1 不同锡前驱体制备的Mo1Sn20催化剂上甲醇氧化的反应性能
Table 1 Reaction performance of methanol oxidation over Mo1Sn20 catalysts with different Sn precursors
Catalyst CH3OH conversion/% C-mol selectivity/% DMM yield/% MF DME FA DMM COx Mo1Sn20-80℃Sn 15.9 8.7 1.3 0 90.0 0 14.3 Mo1Sn20-200℃Sn 17.7 9.0 2.1 0 88.9 0 15.7 Mo1Sn20-300℃Sn 19.5 6.8 2.0 0 91.2 0 17.8 Mo1Sn20-400℃Sn 21.4 5.6 2.1 0 92.3 0 19.8 Mo1Sn20-500℃Sn 21.5 7.1 2.3 0 90.6 0 19.5 Mo1Sn20-600℃Sn 30.0 5.8 4.2 0 90.0 0 27.0 Reaction conditions: atmospheric pressure, tR=140 ℃,n(CH3OH): n(O2)=1∶9.415, CH3OH flow rate=0.817 mL/h, GHSV=7200 h−1. 表 2 不同锡前驱体的织构性能
Table 2 Textural performance of the different Sn precursors
Catalyst Surface area/
(m2·g−1)Volume of pore/
(cm3·g−1)Pore size/
nm80℃Sn 254.65 0.12 1.87 200℃Sn 284.28 0.13 1.83 300℃Sn 212.76 0.13 2.51 400℃Sn 131.87 0.13 4.0 500℃Sn 66.75 0.12 7.2 600℃Sn 37.41 0.12 13.0 表 3 不同锡前驱体制备的Mo1Sn20催化剂的织构性能
Table 3 Textural performance of the Mo1Sn20 catalysts with different Sn precursors
Catalyst Surface area/
(m2·g−1)Volume of pore/
(cm3·g−1)Pore size/
nmMo1Sn20-80℃Sn 112.09 0.12 4.12 Mo1Sn20-200℃Sn 112.65 0.10 3.70 Mo1Sn20-300℃Sn 128.55 0.11 3.39 Mo1Sn20-400℃Sn 85.53 0.10 4.86 Mo1Sn20-500℃Sn 51.64 0.11 8.66 Mo1Sn20-600℃Sn 21.76 0.10 18.45 表 4 不同锡前驱体制备的Mo1Sn20催化剂的XPS-Mo 3d拟合
Table 4 The XPS-Mo 3d fitting results of the Mo1Sn20 catalysts with different Sn precursors
Catalyst Mo6+/% Mo5+/% MoSn20-80℃Sn 49.2 50.8 Mo1Sn20-500℃Sn 76.0 24.0 Mo1Sn20-600℃Sn 79.9 20.1 -
[1] WU Q, TU K, ZENG Y, et al. Discussion on the main problems and countermeasures for building an upgrade version of main energy (coal) industry in China[J]. J China Coal Soc,2019,44(6):1625−1636. [2] XIE H, WU L, ZHENG D. Prediction on the energy consumption and coal demand of China in 2025[J]. J China Coal Soc,2019,44(7):1949−1960. [3] SHIH C F, ZHANG T, LI J H, et al. Powering the future with liquid sunshine[J]. Joule,2018,2(10):1925−1949. doi: 10.1016/j.joule.2018.08.016 [4] REN J, XIN F, XU Y S. A review on direct synthesis of dimethoxymethane[J]. Chin J Chem Eng,2022,50:43−55. doi: 10.1016/j.cjche.2022.09.008 [5] SCHMITZ N, BURGER J, HASSE H. Reaction kinetics of the formation of poly(oxymethylene) dimethyl ethers from formaldehyde and methanol in aqueous solutions[J]. Ind Eng Chem Res,2015,54(50):12553−12560. doi: 10.1021/acs.iecr.5b04046 [6] YUAN M, DONG M R, TIAN Z W, et al. Reaction mechanisms and catalysis in the one-step synthesis of methylal via methanol oxidation[J]. J Energy Inst,2022,103:47−53. doi: 10.1016/j.joei.2022.05.006 [7] GAO X J, ZHANG J F, SONG F E, et al. Selective oxidation conversion of methanol/dimethyl ether[J]. Chem Commun,2022,58(30):4687−4699. doi: 10.1039/D1CC07276E [8] AHMAD W, CHAN F L, SHROTRI A, et al. Dimethoxymethane production via CO2 hydrogenation in methanol over novel Ru based hierarchical BEA[J]. J Energy Chem,2022,66:181−189. doi: 10.1016/j.jechem.2021.07.026 [9] YUAN Y Z, LIU H C, IMOTO H, et al. Performance and characterization of a new crystalline SbRe2O6 catalyst for selective oxidation of methanol to methylal[J]. J Catal,2000,195(1):51−61. doi: 10.1006/jcat.2000.2990 [10] LIU H C, IGLESIA E. Effects of support on bifunctional methanol oxidation pathways catalyzed by polyoxometallate Keggin clusters[J]. J Catal,2004,223(1):161−169. doi: 10.1016/j.jcat.2004.01.012 [11] ZHAO H Y, BENNICI S, SHEN J Y, et al. Nature of surface sites of V2O5-TiO2/ $ {\rm{SO}}_4^{2-} $ catalysts and reactivity in selective oxidation of methanol to dimethoxymethane[J]. J Catal,2010,272(1):176−189. doi: 10.1016/j.jcat.2010.02.028[12] GORNAY J, SECORDEL X, TESQUET G, et al. Direct conversion of methanol into 1, 1-dimethoxymethane: remarkably high productivity over an FeMo catalyst placed under unusual conditions[J]. Green Chem,2010,12(10):1722−1725. doi: 10.1039/c0gc00194e [13] MENG Y L, WAG T, CHEN S, et al. Selective oxidation of methanol to dimethoxymethane on V2O5-MoO3/gamma-Al2O3 catalysts[J]. Appl Catal B: Environ,2014,160:161−172. [14] YUAN Y Z, SHIDO T, IWASAWA Y. The new catalytic property of supported rhenium oxides for selective oxidation of methanol to methylal[J]. Chem Commun,2000,(15):1421−1422. doi: 10.1039/b003870i [15] GUO H Q, LI D B, CHEN C B, et al. The one-step oxidation of methanol to dimethoxymethane over sulfated vanadia-titania catalysts: influence of calcination temperature[J]. RSC Adv,2015,5(79):64202−64207. doi: 10.1039/C5RA09072E [16] THAVORNPRASERT K A, CAPRON M, JALOWIECKI-DUHAMEL L, et al. Highly productive iron molybdate mixed oxides and their relevant catalytic properties for direct synthesis of 1, 1-dimethoxymethane from methanol[J]. Appl Catal B: Environ,2014,145:126−135. doi: 10.1016/j.apcatb.2013.01.043 [17] ZHANG Z Z, ZHANG Q D, JIA L Y, et al. The effects of the Mo-Sn contact interface on the oxidation reaction of dimethyl ether to methyl formate at a low reaction temperature[J]. Catal Sci Technol,2016,6(15):6109−6117. doi: 10.1039/C6CY00460A [18] VALENTE N G, ARRUA L A, CADUS L E. Structure and activity of Sn-Mo-O catalysts: partial oxidation of methanol[J]. Appl Catal A: Gen,2001,205(1-2):201−214. doi: 10.1016/S0926-860X(00)00565-2 [19] LIU H C, CHEUNG P, IGLESIA E. Structure and support effects on the selective oxidation of dimethyl ether to formaldehyde catalyzed by MoOx domains[J]. J Catal,2003,217(1):222−232. [20] GONCALVES F, MEDEIROS P R S, EON J G, et al. Active sites for ethanol oxidation over SnO2-supported molybdenum oxides[J]. Appl Catal A: Gen,2000,193(1/2):195−202. doi: 10.1016/S0926-860X(99)00430-5 [21] 熊盼, 高秀娟, 王文秀, 等. 焙烧温度对钼锡催化剂结构和二甲醚氧化性能的影响[J]. 燃料化学学报,2022,50(1):63−71.XIONG Pan, GAO Xiu-juan, WANG Wen-xiu, et al. Effect of calcination temperature on the structure and performance of molybdenum-tin catalyst for DME oxidation[J]. J Fuel Chem Technol,2022,50(1):63−71. [22] 王文秀. 钼基催化剂上甲醇低温氧化研究[D]. 北京: 中国科学院大学, 2021.WANG Wen-xiu. Study on low temperature oxidation of methanol over molybdenum-based catalyst[D]. Beijing: University of Chinese Academy of Sciences, 2021. [23] YAN L N, ZHANG J F, GAO X J, et al. Oxidative coupling of methane over Mo-Sn catalysts[J]. Chem Commun,2021,57(98):13297−13300. doi: 10.1039/D1CC04821J [24] CHIORINO A, GHIOTTI G, PRINETTO F, et al. Preparation and characterization of SnO2 and MoOx-SnO2 nanosized powders for thick film gas sensors[J]. Sens Actuators B: Chem,1999,58(1/3):338−349. doi: 10.1016/S0925-4005(99)00094-5 [25] MAKEEVA E A, RUMYANTSEVA M N, GAS'KOV A M. Synthesis, microstructure, and gas-sensing properties of SnO2/MoO3 nanocomposites[J]. Inorg Mater,2005,41(4):370−377. doi: 10.1007/s10789-005-0139-4 [26] SHAKIR I, SHAHID M, CHEREVKO S, et al. Ultrahigh-energy and stable supercapacitors based on intertwined porous MoO3-MWCNT nanocomposites[J]. Electrochim Acta,2011,58:76−80. doi: 10.1016/j.electacta.2011.08.076 [27] ZHANG Z Z, ZHANG Q D, JIA L Y, et al. Effects of tetrahedral molybdenum oxide species and MoOx domains on the selective oxidation of dimethyl ether under mild conditions[J]. Catal Sci Technol,2016,6(9):2975−2983. doi: 10.1039/C5CY01569C [28] 杨奇, 高秀娟, 冯茹, 等. 水热合成的MoO3-SnO2催化剂催化氧化二甲醚的性能研究[J]. 燃料化学学报,2019,47(8):934−941. doi: 10.1016/S1872-5813(19)30038-6YANG Qi, GAO Xiu-juan, FENG Ru, et al. MoO3-SnO2 catalyst prepared by hydrothermal synthesis method for dimethyl ether catalytic oxidation[J]. J Fuel Chem Technol,2019,47(8):934−941. doi: 10.1016/S1872-5813(19)30038-6 [29] 王文秀, 高秀娟, 熊盼, 等. Mo-Sn催化剂上甲醇低温氧化制甲缩醛[J]. 燃料化学学报,2021,49(10):1487−1494. doi: 10.1016/S1872-5813(21)60094-4WANG Wen-xiu, GAO Xiu-juan, XIONG Pan, et al. Low-temperature oxidation of methanol over Mo-Sn catalyst[J]. J Fuel Chem Technol,2021,49(10):1487−1494. doi: 10.1016/S1872-5813(21)60094-4 [30] GU Y Y, ZHANG Z Z, WANG W F, et al. Effects of calcination atmosphere on the structure and performance of MoO3-SnO2 catalyst for theoxidation of dimethyl ether at low temperature[J]. J Fuel Chem Technol,2017,45(5):572−580. [31] YUAN M, TANG R Y, SUN X Y, et al. Effects of the support on bifunctional one-step synthesis of methylal via methanol oxidation catalysed by Fe-Mo-based bifunctional catalysts[J]. Sustainable Energy Fuels,2021,5(1):246−260. doi: 10.1039/D0SE01194K [32] ROJAS F, KORNHAUSER I, FELIPE C, et al. Capillary condensation in heterogeneous mesoporous networks consisting of variable connectivity and pore-size correlation[J]. Phys Chem Chem Phys,2002,4(11):2346−2355. doi: 10.1039/b108785a [33] 熊盼. 二甲醚低温氧化钼基催化剂活性位结构与性能的研究[D]. 北京: 中国科学院大学, 2021.XIONG Pan. Study on the structure and properties of active site of low temperature oxidation of dimethyl ether over molybdenum based catalyst[D]. Beijing: University of Chinese Academy of Sciences, 2021. [34] MESTL G, SRINIVASAN T K K. Raman spectroscopy of monolayer-type catalysts: Supported molybdenum oxides[J]. Catal Rev,1998,40(4):451−570. doi: 10.1080/01614949808007114 [35] YANG J K, ZHAO H L, ZHANG F C. Effects of heat treatment on the chemical states of O 1s and Sn 3d at the surface of SnOx: F films by APCVD[J]. Mater Lett,2013,90:37−40. doi: 10.1016/j.matlet.2012.07.055 [36] TAN X J, WANG L Z, CHENG C, et al. Plasmonic MoO3-x@MoO3 nanosheets for highly sensitive SERS detection through nanoshell-isolated electromagnetic enhancement[J]. Chem Commun,2016,52(14):2893−2896. doi: 10.1039/C5CC10020H [37] YANG J, XIAO X, CHEN P, et al. Creating oxygen-vacancies in MoO3-x nanobelts toward high volumetric energy-density asymmetric supercapacitors with long lifespan[J]. Nano Energy,2019,58(1):455−465. -





 下载:
下载: