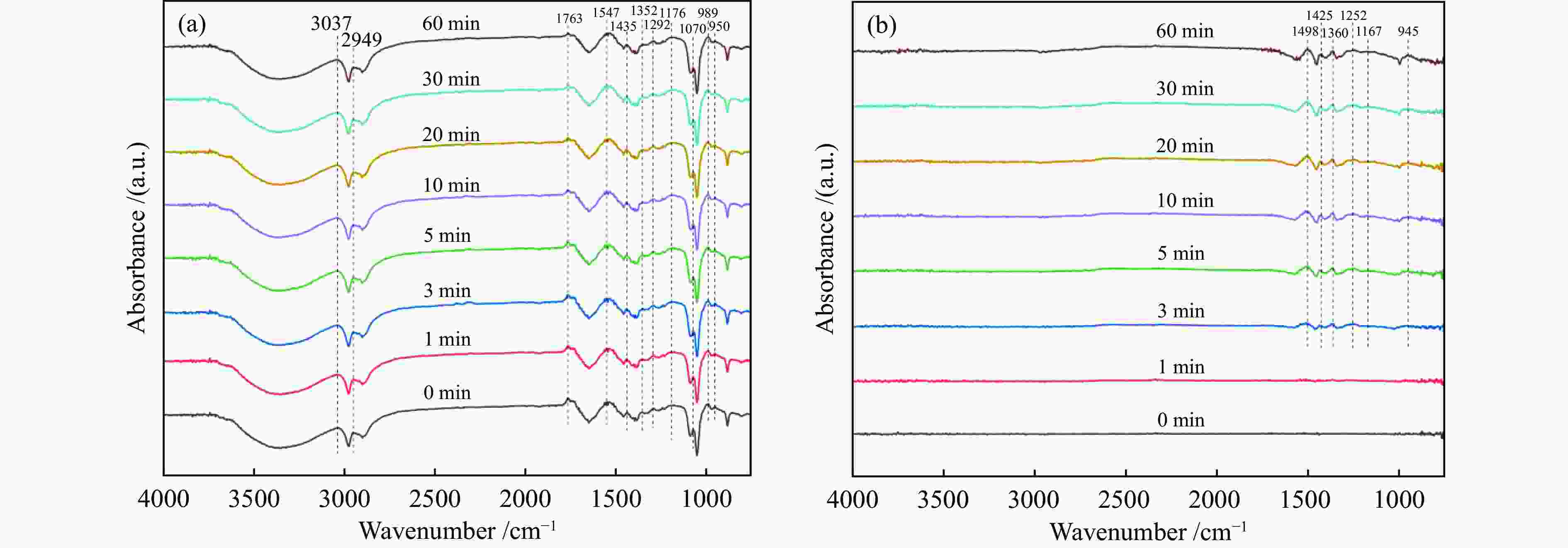
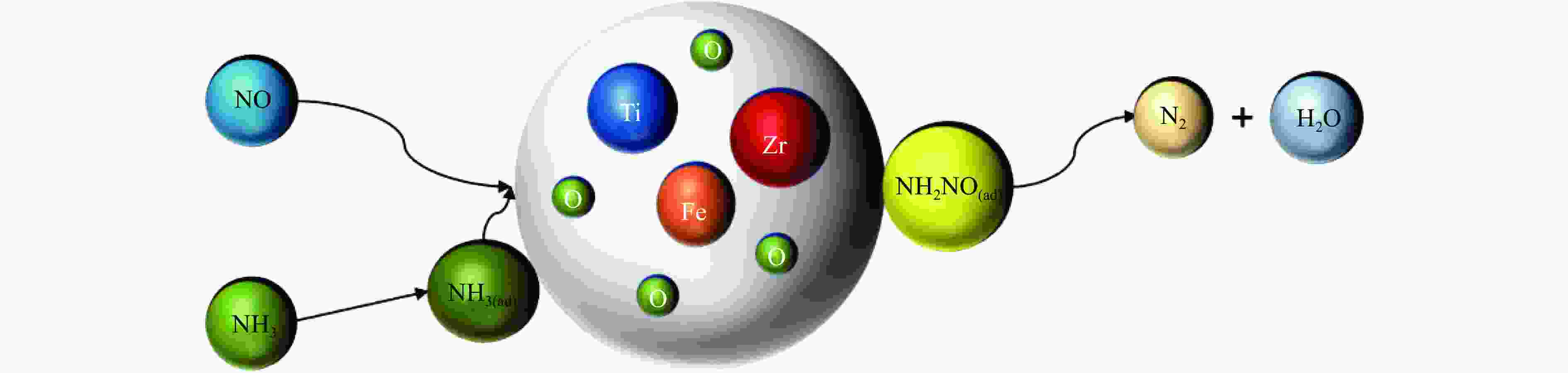
-
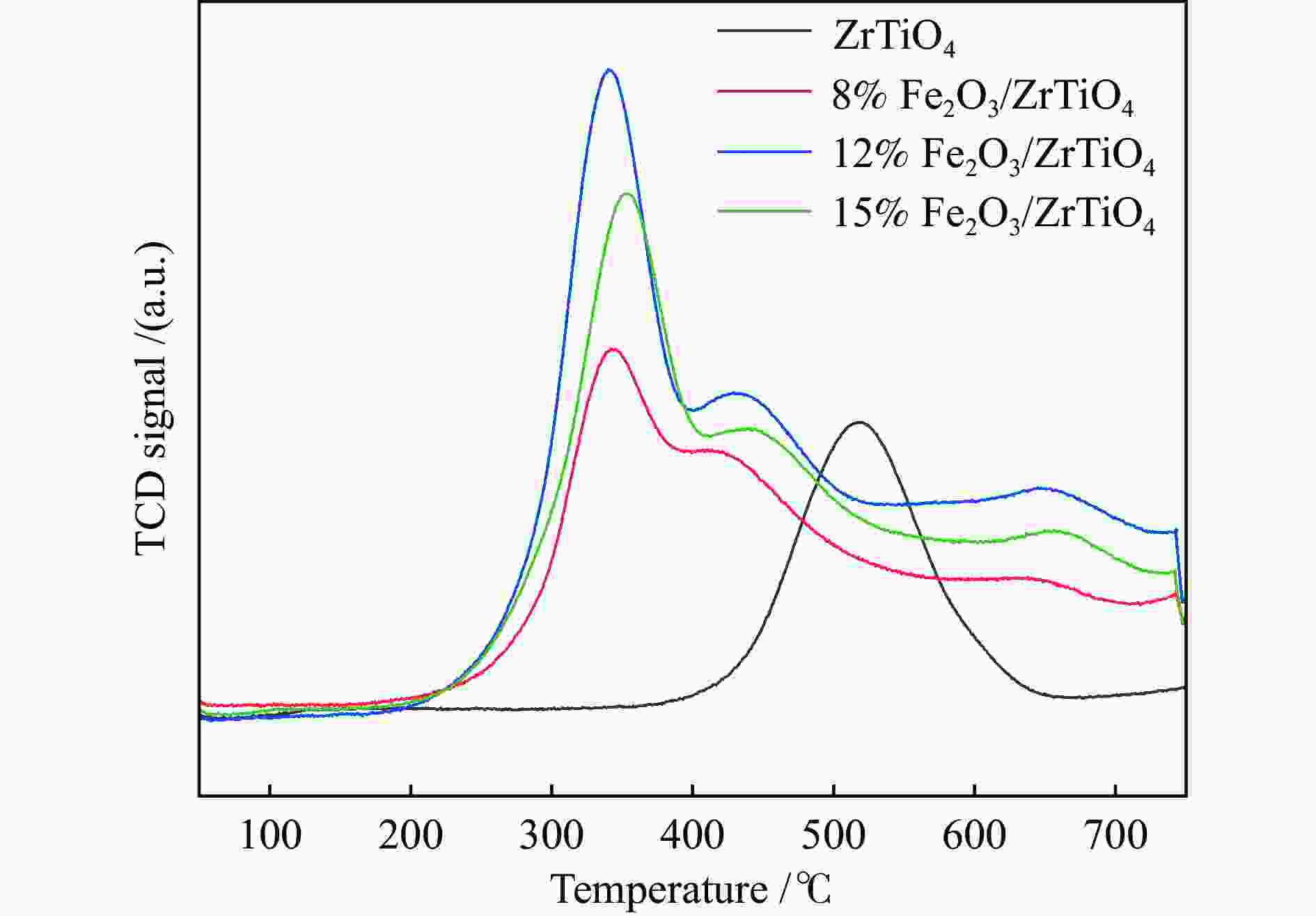
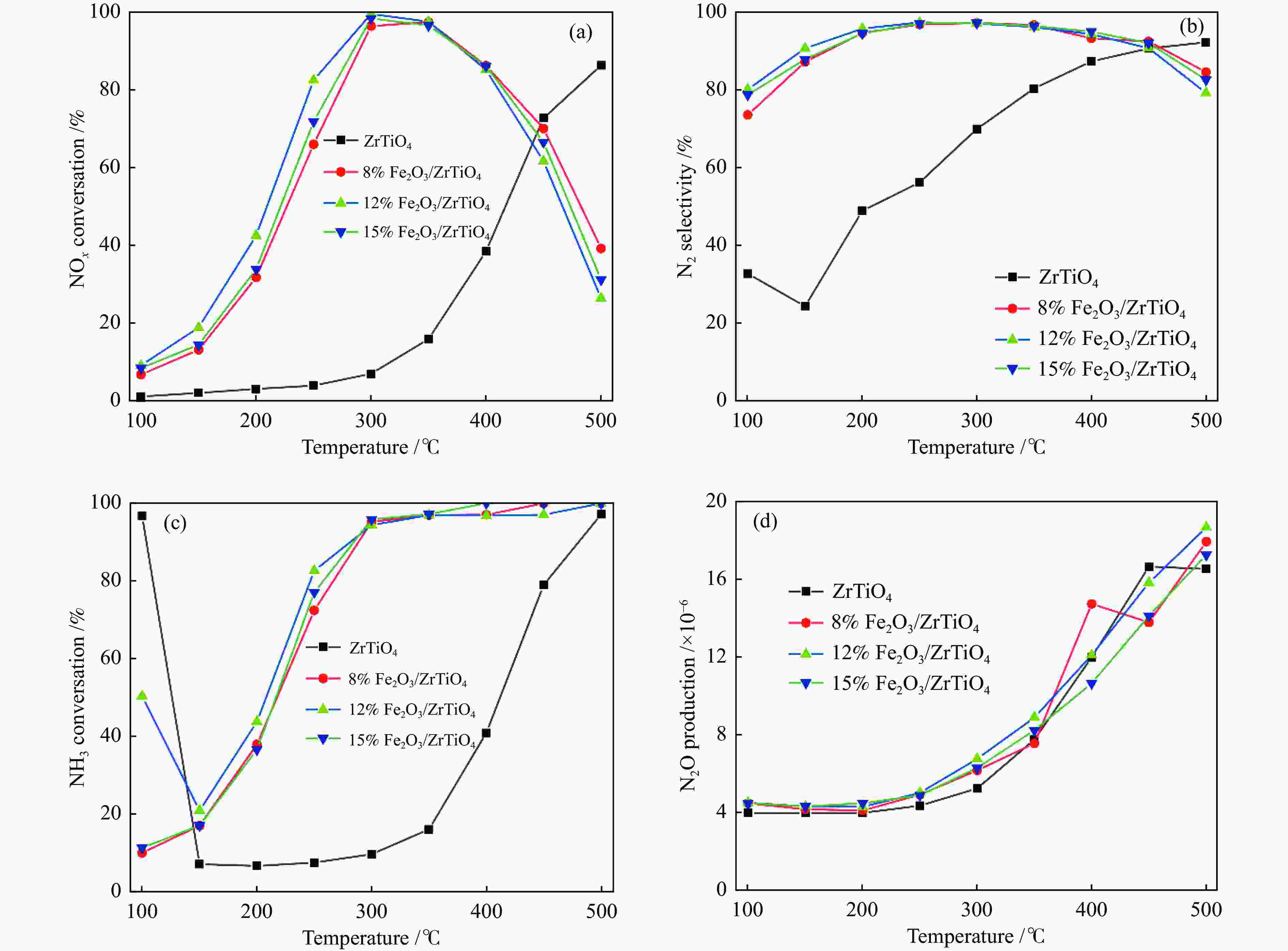
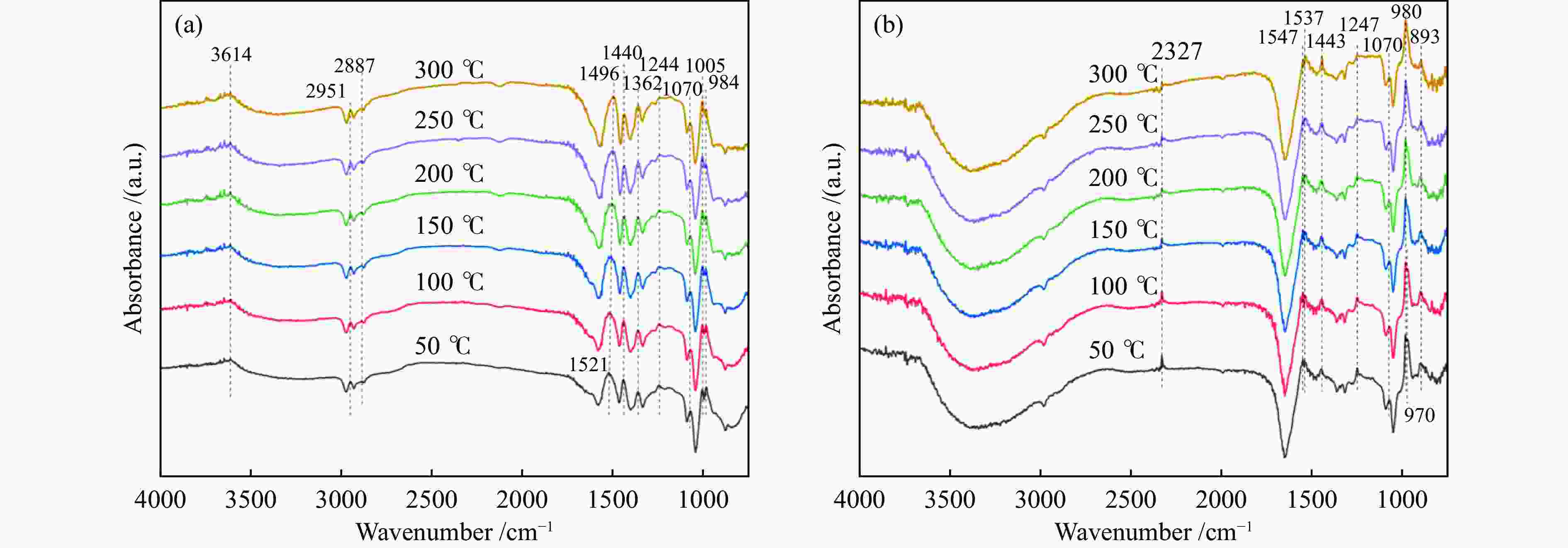
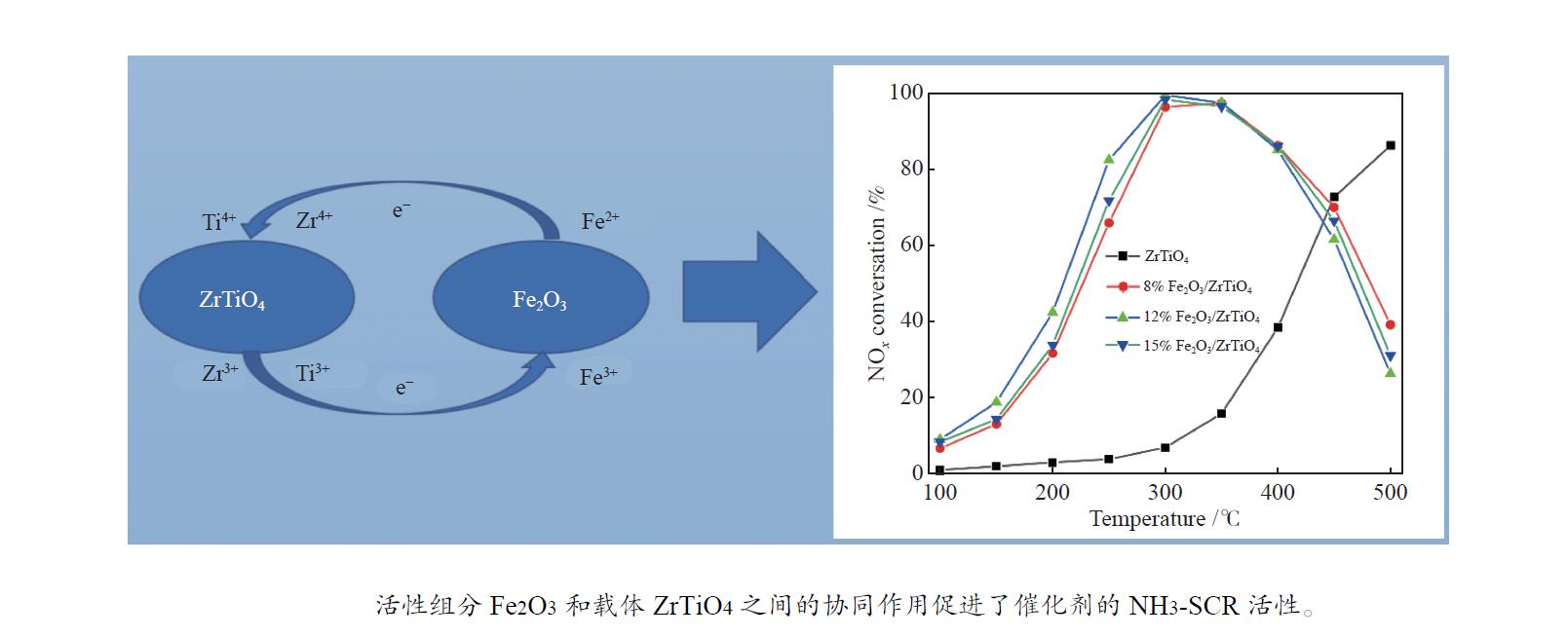
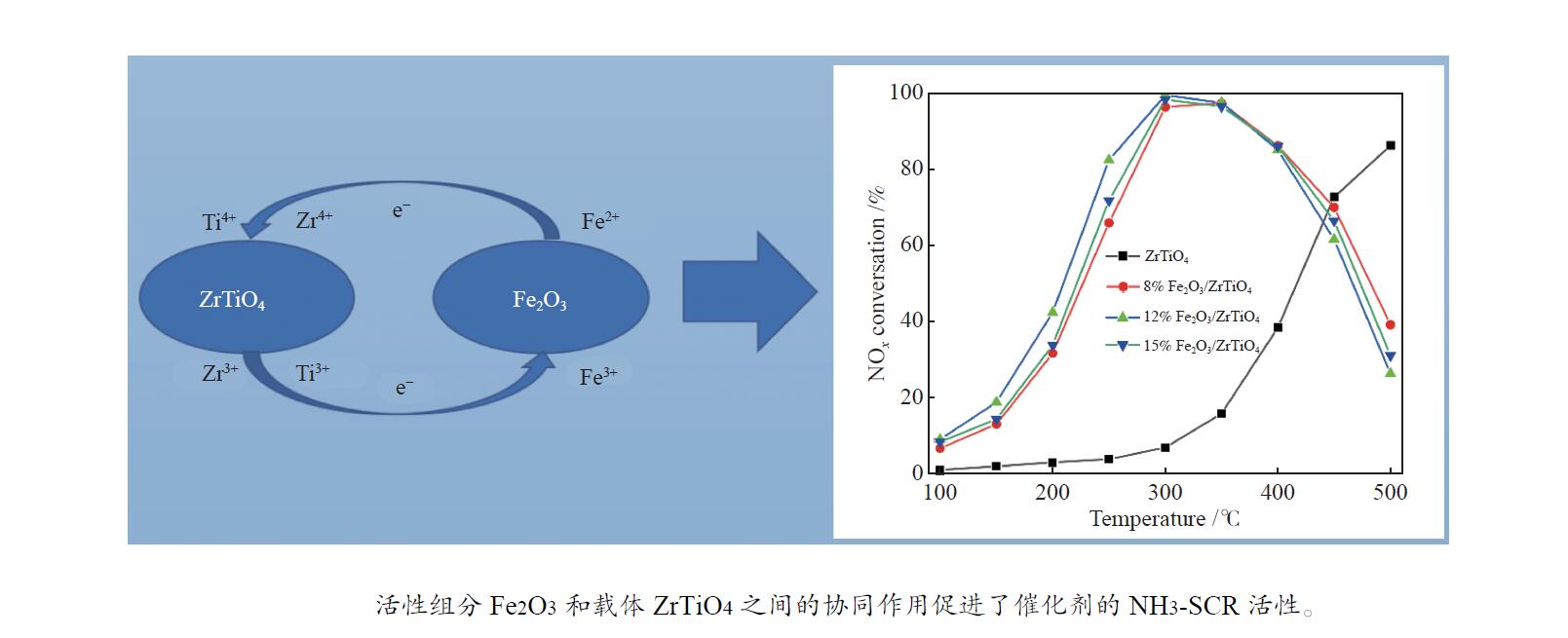
摘要: NH3-SCR催化剂主要用于工业生产和汽车尾气清洁,本研究采用“共沉淀-浸渍法”制备了新型α%Fe2O3/ZrTiO4(α=0、8、12、15)催化剂。结果表明,α%Fe2O3/ZrTiO4催化剂的最佳成分配比的12%Fe2O3/ZrTiO4催化剂在250−400 ℃条件下NOx转化率大于80%,在300 ℃时NOx转化率接近100%,并且N2选择性在200−450 ℃大于90%。在ZrTiO4表面负载Fe2O3后,催化剂的氧化还原性能、表面酸度和Oβ/(Oα + Oβ)比例都有所提高,这不仅归因于α%Fe2O3/ZrTiO4催化剂具有多孔结构,还归因于活性组分Fe2O3和载体ZrTiO4之间的电子相互作用。此外,原位DRIFTs反应表明,12%Fe2O3/ZrTiO4催化剂的NH3-SCR反应遵循Eley-Rideal机制。明确的反应机制有利于更深入了解SCR过程中NOx转化的反应过程。这项工作为未来Fe基SCR催化剂在中温范围内替代V基催化剂提供了可行的策略。
-
关键词:
- Fe2O3/ZrTiO4催化剂 /
- NH3-SCR /
- 多孔 /
- 反应机制
Abstract: The selective catalytic reduction (SCR) NH3 catalyst is mainly used in industrial production and automobile exhaust cleaning. In this study, a novel α%Fe2O3/ZrTiO4 (α=0, 8, 12, 15) catalyst was prepared by the coprecipitation impregnation method. The results show that the NOx conversion rate of 12%Fe2O3/ZrTiO4 catalyst with the optimal composition is high above 80% at 250−400 °C, close to 100% at 300 °C, and N2 selectivity is high above 90% at 200−450 °C. The redox properties, surface acidity, and Oβ/(Oα + Oβ) ratio of ZrTiO4 catalysts are improved after loading Fe2O3 on the ZrTiO4 surface, which is attributed not only to the porous structure of α%Fe2O3/ZrTiO4 catalyst but also to the synergistic interaction between the active component Fe2O3 and the support ZrTiO4. In addition, in-situ DRIFT reactions show that the NH3-SCR reaction of 12%Fe2O3/ZrTiO4 catalyst follows the Eley-Rideal mechanism. A clear reaction mechanism is conducive to a deeper understanding of the reaction process of NOx conversion during SCR. This work provides a feasible strategy for Fe-based SCR catalysts to replace V-based catalysts in the medium temperature range in the future.-
Key words:
- Fe2O3/ZrTiO4 catalyst /
- NH3-SCR /
- porous structure /
- reaction mechanism
-
Table 1 SBET surface area, pore volume and pore size of ZrTiO4 and α%Fe2O3/ZrTiO4 samples
Sample Surface area
/ (m2·g−1)Pore volume
/(cm3·g−1)Pore size
/nmZrTiO4 161.3 0.85 21.19 8%Fe2O3/ZrTiO4 134.3 0.50 14.87 12%Fe2O3/ZrTiO4 139.6 0.77 22.02 15%Fe2O3/ZrTiO4 129.1 0.58 17.92 Table 2 XPS concentration of ZrTiO4 and α%Fe2O3/ZrTiO4
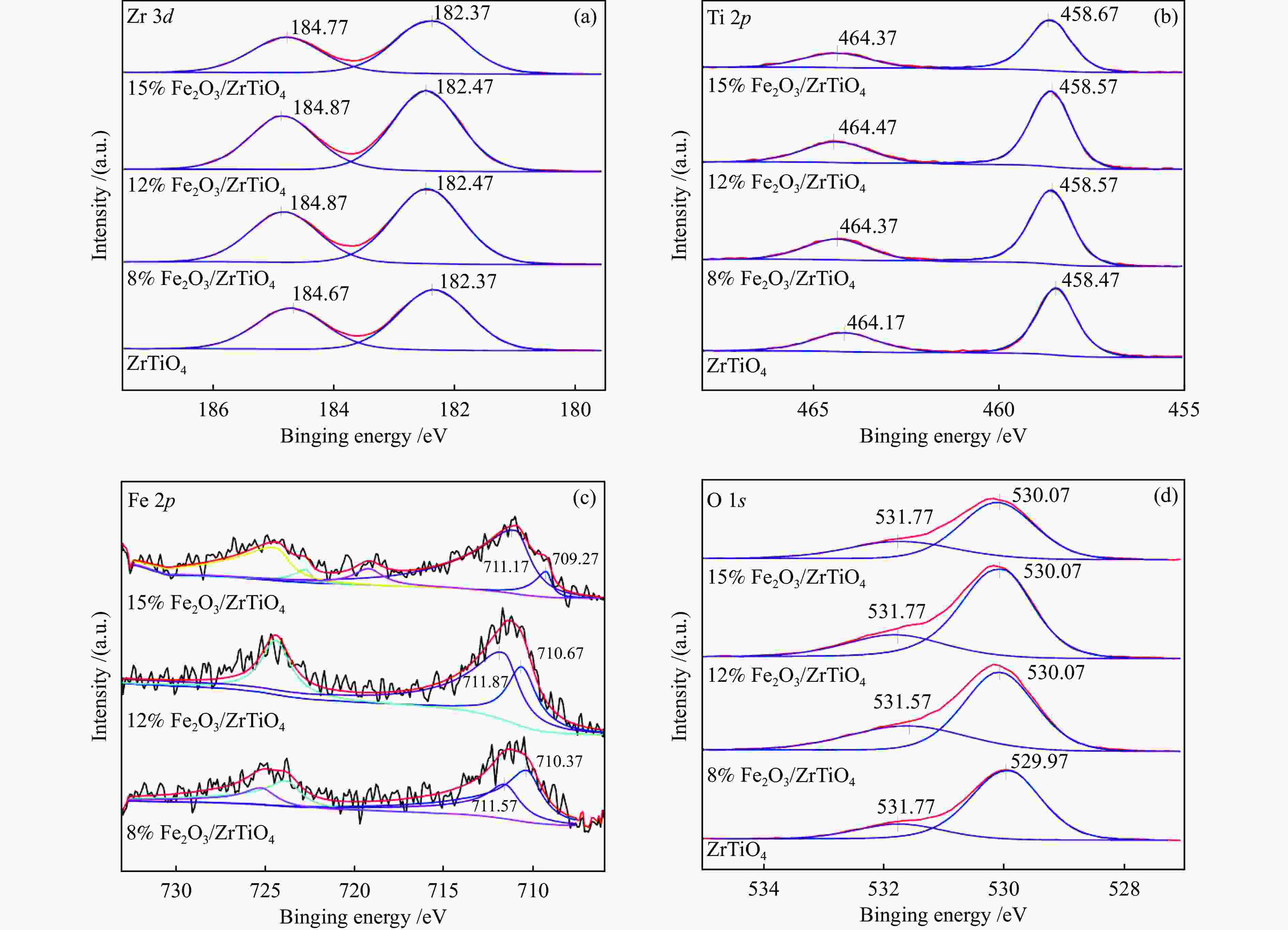
Sample Atomic concentration Relative concentration /% Fe Zr Ti O Oβ/(Oα + Oβ) Fe3+ /(Fe3+ + Fe2+ ) ZrTiO4 − 14.4 15.3 70.3 23.1 − 8%Fe2O3/ZrTiO4 1.85 13.3 13.2 71.6 34.0 43 12%Fe2O3/ZrTiO4 2.21 13.3 12.8 71.6 31.7 73.4 15%Fe2O3/ZrTiO4 2.68 13.2 13.1 71.0 28.7 91.7 -
[1] ROY B, CHOO W L, BHATTACHARYA S. Prediction of distribution of trace elements under oxy-fuel combustion condition using Victorian brown coals[J]. Fuel,2013,114:135−142. doi: 10.1016/j.fuel.2012.09.080 [2] GANESH C D, SUBHASHISH D, DEVENDRA M, RAM P. Study of Fe, Co, and Mn-based perovskite-type catalysts for the simultaneous control of soot and NOx from diesel engine exhaust[J]. Mater Dis,2017,10:37−42. doi: 10.1016/j.md.2018.04.002 [3] SEINFELD J H. Urban air pollution: State of the science[J]. Science,1989,243(4892):745−752. doi: 10.1126/science.243.4892.745 [4] LIU Z M, HAO J M, FU L X, ZHU T L. Study of Ag/La0.6Ce0.4CoO3 catalysts for direct decomposition and reduction of nitrogen oxides with propene in the presence of oxygen[J]. Appl Catal B: Environ,2003,44(4):355−370. doi: 10.1016/S0926-3373(03)00103-6 [5] TOPSOE N Y. Mechanism of the selective catalytic reduction of nitric oxide by ammonia elucidated byin situ on-line Fourier transform infrared spectroscopy[J]. Science,1994,265(5176):1217−1219. doi: 10.1126/science.265.5176.1217 [6] CHEN L, LI J H, GE M F. Promotional effect of Ce-doped V2O5-WO3/TiO2 with low vanadium loadings for selective catalytic reduction of NOx by NH3[J]. J Phys Chem C,2009,113(50):21177−21184. doi: 10.1021/jp907109e [7] LONG R Q, YANG R T. Superior Fe-ZSM-5 catalyst for selective catalytic reduction of nitric oxide by ammonia[J]. J Am Chem Soc,1999,121(23):5595−5596. doi: 10.1021/ja9842262 [8] LIAN Z H, LIU F D, HE H. Enhanced activity of Ti-modified V2O5/CeO2 catalyst for the selective catalytic reduction of NOx with NH3[J]. Ind Eng Chem Res,2014,53(50):19506−19511. [9] HUANG T J, ZHANG Y P, ZHUANG K, LU BIN, ZHU YIWEN, SHEN KAI. Preparation of honeycombed holmium-modified Fe-Mn/TiO2 catalyst and its performance in the low temperature selective catalytic reduction of NOx[J]. J Fuel Chem Technol,2018,46(3):319−327. doi: 10.1016/S1872-5813(18)30015-X [10] BIE X, WU K, JIAO K L, ZHAO K, CHEN X Y, MA S C. Behavior and structure tuning of (Mn&Fe)AlOx-based catalysts for superior denitrification performance[J]. J Environ Chem Eng,2021,9(5):106153. doi: 10.1016/j.jece.2021.106153 [11] FOO R, VAZHNOVA T, LUKYANOV D B, MILLINGTON P, COLLIER J, RAJARAM R, GOLUNSKI S. Formation of reactive Lewis acid sites on Fe/WO3-ZrO2 catalysts for higher temperature SCR applications[J]. Appl Catal B: Environ,2015,162:174−179. doi: 10.1016/j.apcatb.2014.06.034 [12] FAN B Y, ZHANG Z Y, LIU C X, LIU Q L. Investigation of sulfated iron-based catalysts with different sulfate position for selective catalytic reduction of NOx with NH3[J]. Catalysts,2020,10(9):1035. doi: 10.3390/catal10091035 [13] HUANG H F, CHEN Y J, YANG R, ZHU Q L, LU H F. Fe-V/TiO2 catalysts for selective catalytic reduction of NOx with NH3 in diesel exhaust[J]. J Fuel Chem Technol,2014,42(6):751−757. [14] ZHOU Y Y, XIE Z Y, JIANG J X, WANG J, SONG X Y, HE Q, DING W, WEI Z D. Lattice-confined Ru clusters with high CO tolerance and activity for the hydrogen oxidation reaction[J]. Nat Catal,2021,4(4):341−341. doi: 10.1038/s41929-021-00601-5 [15] WEI X L, ZHAO R Q, CHU B X, XIE S Z, QIN Q J, CHEN K, LI L L, ZHAO S L, BIN LI, DONG L H. Significantly enhanced activity and SO2 resistance of Zr-modified CeTiOx catalyst for low-temperature NH3-SCR by H2 reduction treatment[J]. Mol Catal,2022,518:112069. doi: 10.1016/j.mcat.2021.112069 [16] LIU C X, BI Y L, LI J H. Activity enhancement of sulphated Fe2O3 supported on TiO2-ZrO2 for the selective catalytic reduction of NO by NH3[J]. Appl Surf Sci,2020,528:146695. doi: 10.1016/j.apsusc.2020.146695 [17] GUO K, JI J W, OSUGA R, ZHU Y X, SUN J F, TANG C J, KONDO J N, DONG L. Construction of Fe2O3 loaded and mesopore confined thin-layer titania catalyst for efficient NH3-SCR of NOx with enhanced H2O/SO2 tolerance[J]. Appl Catal B: Environ,2021,287:119982. doi: 10.1016/j.apcatb.2021.119982 [18] GONG Z Q, NIU S L, ZHANG Y J, LU C M. Facile synthesis of porous α-Fe2O3 nanostructures from MIL-100(Fe) via sacrificial templating method, as efficient catalysts for NH3-SCR reaction[J]. Mater Res Bull,2020,123:110693. doi: 10.1016/j.materresbull.2019.110693 [19] FANG N J, GUO J X, SHU S, LUO H D, CHU Y H, LI J J. Enhancement of low-temperature activity and sulfur resistance of Fe0.3Mn0.5Zr0.2 catalyst for NO removal by NH3-SCR[J]. Chem Eng J,2017,325:114−123. doi: 10.1016/j.cej.2017.05.053 [20] LIU F D, SHAN W P, LIAN Z H, LIU J J, HE H. The smart surface modification of Fe2O3 by WOx for significantly promoting the selective catalytic reduction of NOx with NH3[J]. Appl Catal B: Environ,2018,230:165−176. doi: 10.1016/j.apcatb.2018.02.052 [21] ZHAO K, MENG J P, LU J Y, HE Y, HUANG H Z, TANG Z C, ZHEN X P. Sol-gel one-pot synthesis of efficient and environmentally friendly iron-based catalysts for NH3-SCR[J]. Appl Surf Sci,2018,445:454−461. doi: 10.1016/j.apsusc.2018.03.160 [22] HOU Y Q, WANG J C, LI Q Y, LIU Y J, BAI Y R, ZENG Z Q, HUANG Z G. Environmental-friendly production of FeNbTi catalyst with significant enhancement in SCR activity and SO2 resistance for NOx removal[J]. Fuel,2021,285:119133. doi: 10.1016/j.fuel.2020.119133 [23] REN D D, GUI K T, GU S C, WEI Y L. Mechanism of improving the SCR NO removal activity of Fe2O3 catalyst by doping Mn[J]. J Alloys Comp,2021,867:158787. doi: 10.1016/j.jallcom.2021.158787 [24] SHEN B X, WANG Y Y, WANG F M, LIU T. The effect of Ce-Zr on NH3-SCR activity over MnOx(0.6)/Ce0.5Zr0.5O2 at low temperature[J]. Chem Eng J,2014,236:171−180. doi: 10.1016/j.cej.2013.09.085 [25] SWIRK K, WANG Y, HU C W, LI L, DA C P, DELAHAY G. Novel preparation of Cu and Fe zirconia supported catalysts for selective catalytic reduction of NO with NH3[J]. Catalysts,2021,11(1):55. doi: 10.3390/catal11010055 [26] LI C X, XIONG Z B, HE J F, QU X K, LI Z Z, NING X, LU W, WU S M, TAN L Z. Influence of ignition atmosphere on the structural properties of magnetic iron oxides synthesized via solution combustion and the NH3-SCR activity of W/Fe2O3 catalyst[J]. Appl Catal A: Gen,2020,602:117726. doi: 10.1016/j.apcata.2020.117726 [27] LI Y F, HOU Y Q, ZHANG Y Z, YANG Y T, HUANG Z G. Confinement of MnOx@Fe2O3 core-shell catalyst with titania nanotubes: Enhanced N2 selectivity and SO2 tolerance in NH3-SCR process[J]. J Colloid Interface Sci,2021,608:2224−2234. [28] ZHOU X, YU F, SUN R B, TIAN J Q, WANG Q, DAI B, DAN J M, PFEIFFER H. Two-dimensional MnFeCo layered double oxide as catalyst for enhanced selective catalytic reduction of NOx with NH3 at low temperature (25-150 degrees C)[J]. Appl Catal A: Gen,2020,592:117432. doi: 10.1016/j.apcata.2020.117432 [29] SONG L, MA K, TIAN W, JI J Y, LIU C J, TANG S Y, JIANG W, YUE H R, LIANG B. An environmentally friendly FeTiSOx catalyst with a broad operation-temperature window for the NH3-SCR of NOx[J]. AICHE J,2019,65(10):e16684. [30] XU L T, NIU S L, LU C M, ZHANG Q, LI J. Influence of calcination temperature on Fe0.8Mg0.2Oz catalyst for selective catalytic reduction of NOx with NH3[J]. Fuel,2018,219:248−258. doi: 10.1016/j.fuel.2018.01.083 [31] WANG X B, ZHANG L, WU S G, ZOU W X, YU S H, SHAO Y, DONG L. Promotional effect of Ce on iron-based catalysts for selective catalytic reduction of NO with NH3[J]. Catalysts,2016,6(8):112. doi: 10.3390/catal6080112 [32] XIONG Z B, WU C, HU Q, WANG Y Z, JIN J, LU C M, GUO D X. Promotional effect of microwave hydrothermal treatment on the low-temperature NH3-SCR activity over iron-based catalyst[J]. Chem Eng J,2016,286:459−466. doi: 10.1016/j.cej.2015.10.082 [33] ZHOU Y H, REN S, WANG M M, YANG J, CHEN Z C, CHEN L. Mn and Fe oxides co-effect on nanopolyhedron CeO2 catalyst for NH3-SCR of NO[J]. J Energy Inst,2021,99:97−104. doi: 10.1016/j.joei.2021.08.003 [34] WU Z B, JIANG B Q, LIU Y, WANG H Q, JIN R B. DRIFT study of manganese/ titania-based catalysts for low-temperature selective catalytic reduction of NO with NH3[J]. Environ Sci Technol,2007,41(16):5812−7. [35] GUAN B, LIN H, ZHU L, HUANG Z. Selective catalytic reduction of NOx with NH3 over Mn, Ce substitution Ti0.9V0.1O2-delta nanocomposites catalysts prepared by self-propagating high-temperature synthesis method[J]. J Phys Chem C,2011,115(26):12850−12863. doi: 10.1021/jp112283g [36] JIANG B Q, LI Z G, LEE S C. Mechanism study of the promotional effect of O2 on low-temperature SCR reaction on Fe-Mn/TiO2 by DRIFT[J]. Chem Eng J,2013,225:52−58. doi: 10.1016/j.cej.2013.03.022 [37] LI X Y, CHEN J, LU C M, LUO G Q, YAO H. Performance of Mo modified γ-Fe2O3 catalyst for selective catalytic reduction of NOx with ammonia: Presence of arsenic in flue gas[J]. Fuel,2021,294:120552. doi: 10.1016/j.fuel.2021.120552 [38] CHEN L, LI J H, GE M F. DRIFT study on cerium-tungsten/titania catalyst for selective catalytic reduction of NOx with NH3[J]. Environ Sci Technol,2010,44(24):9590−9596. [39] HADJIIVANOV K I. Identification of neutral and charged NxOy surface species by ir spectroscopy[J]. Catal Rev,2000,42(1-2):71−144. doi: 10.1081/CR-100100260 [40] LIU F D, HE H, DING Y, ZHANG C B. Effect of manganese substitution on the structure and activity of iron titanate catalyst for the selective catalytic reduction of NO with NH3[J]. Appl Catal B: Environ,2009,93(1):194−204. [41] XU H D, WANG Y, CAO Y, FANG Z T, LIN T, GONG M C, CHEN Y Q. Catalytic performance of acidic zirconium-based composite oxides monolithic catalyst on selective catalytic reduction of NOx with NH3[J]. Chem Eng J,2014,240:62−73. doi: 10.1016/j.cej.2013.11.053 [42] ARTURO M-A, JAVIER S, JOSÉ C C, XOSÉ S, ADOLFO A, RENATO C. NO reaction at surface oxygen vacancies generated in cerium oxide[J]. J Chem Soc, Faraday Trans,1995,91:1679−1687. doi: 10.1039/FT9959101679 [43] FAN Z Y, SHI J W, GAO C, GAO G, WANG B R, NIU C M. Rationally designed porous MnOx-FeOx nanoneedles for low-temperature selective catalytic reduction of NOx by NH3[J]. ACS Appl Mater Interfaces,2017,9(19):16117−16127. [44] MA S B, ZHAO X Y, LI Y S, ZHANG T R, YUAN F L, NIU X Y, ZHU Y J. Effect of W on the acidity and redox performance of the Cu0.02Fe0.2WaTiOx (a = 0.01, 0.02, 0.03) catalysts for NH3-SCR of NO[J]. Appl Catal B: Environ,2019,248:226−238. doi: 10.1016/j.apcatb.2019.02.015 [45] SHU Y, SUN H, QUAN X, CHEN S. Enhancement of catalytic activity over the iron-modified Ce/TiO2 catalyst for selective catalytic reduction of NOx with ammonia[J]. J Phys Chem C,2012,116(48):25319−25327. doi: 10.1021/jp307038q [46] WANG H M, NING P, ZHANG Y Q, MA Y P, WANG J F, WANG L Y, ZHANG Q L. Highly efficient WO3-FeOx catalysts synthesized using a novel solvent-free method for NH3-SCR[J]. J Hazardous Mater,2020,388:121812. doi: 10.1016/j.jhazmat.2019.121812 [47] GAO C, SHI J W, FAN Z Y, WANG B R, WANG Y, He C, WANG X B, LI J, NIU C M. "Fast SCR" reaction over Sm-modified MnOx-TiO2 for promoting reduction of NOx with NH3[J]. Appl Catal A: Gen,2018,564:102−112. doi: 10.1016/j.apcata.2018.07.017 [48] GUI K T, LIANG H, ZHA X B. DRIFTS study of gamma Fe2O3 nano-catalyst for low-temperature selective catalytic reduction of NOx with NH3[J]. Can J Chem Eng,2016,94(9):1668−1675. doi: 10.1002/cjce.22546 -




 下载:
下载: