Effect of surface modification of Fe/g-C3N4 catalyst on the product distribution in CO hydrogenation
-
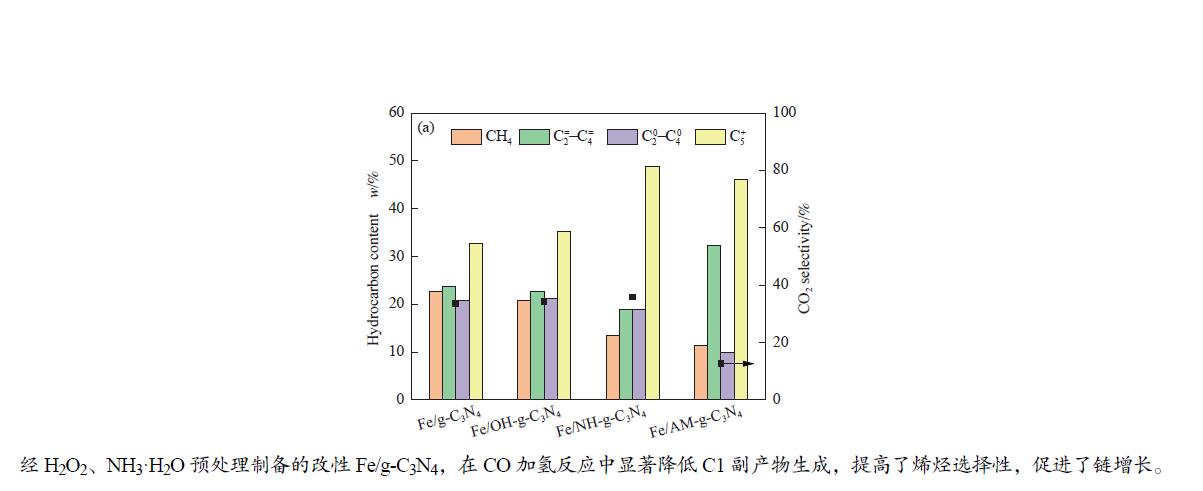
摘要: 采用尿素热缩合法制备了氮化碳(g-C3N4),经H2O2、NH3·H2O处理、浸渍法负载Fe制得改性Fe/g-C3N4,对比研究了改性前后催化剂的CO加氢性能。结合XRD、SEM、FT-IR、CO2-TPD、CO-TPD、H2-TPR、接触角测试和N2物理吸附-脱附等系列表征,探究了表面预处理对Fe/g-C3N4催化剂织构性质以及CO加氢产物分布的影响。结果表明,不同改性方法对催化剂的织构性质和CO加氢性能影响显著。尿素热缩合法制备的g-C3N4具有典型蜂窝状结构,Fe与g-C3N4相互作用较强,且高度分散;改性前后样品均呈亲水性,且H2O2、NH3·H2O处理后亲水性增强,H2O2处理增强了表面羟基,NH3·H2O处理增加了表面氨基,促进了CO吸附,促使Fe(NCN)物相生成;预处理后的催化剂表面碱性增强。在CO加氢反应中,两步改性后的Fe/AM-g-C3N4催化剂,CO2选择性降至11.61%;Fe/AM-g-C3N4表面碱性增强,抑制了烯烃二次加氢,烯烃选择性较高,
${\rm{C}}_2^=-{\rm{C}}_4^= $ 达32.37%,O/P值3.23。-
关键词:
- CO加氢 /
- 表面改性 /
- Fe/g-C3N4催化剂 /
- 产物分布
Abstract: Carbon nitride (g-C3N4) prepared using thermal condensation of urea was pretreated by H2O2/NH3·H2O and used as support to obtain Fe/g-C3N4 catalyst via impregnation method. The catalytic performance of the catalysts both before and after modification was investigated in CO hydrogenation. Combining detailed characterizations, such as XRD, SEM, TEM, FT-IR, TG, CO2-TPD, CO-TPD, H2-TPR, contact angle measurement, and N2 physical adsorption and desorption, we investigated the effects of surface pretreatment on the texture properties of Fe/g-C3N4 catalysts and the product distribution of CO hydrogenation. The results demonstrate that various pretreatment techniques have significant influences on the textural properties and catalytic performance of the catalysts. The prepared g-C3N4 with a typical honeycomb structure has strong interaction with highly dispersed Fe. Both before and after modification, the materials are hydrophilic, and the hydrophilicity is increased after treatment with H2O2 and NH3·H2O. Treatment with H2O2 enhances surface hydroxyl groups. NH3·H2O treatment improves surface amino groups, promotes CO adsorption, and facilitates the formation of Fe(NCN) phase. The surface basicity of all pretreated catalysts is enhanced. The water gas shift (WGS) reaction activity of the two-step modified catalyst Fe/AM-g-C3N4 was lower, and the CO2 selectivity in CO hydrogenation was reduced to 11.61%. Due to the enhanced basicity of Fe/AM-g-C3N4, the secondary hydrogenation ability of olefins was inhibited to obtain higher olefin selectivity with${\rm{C}}_2^=-{\rm{C}}_4^=\;s \;{\rm{of}}$ 32.37% and an O/P value of 3.23.-
Key words:
- CO hydrogenation /
- surface modification /
- Fe/g-C3N4 catalyst /
- product distribution
-
表 1 催化剂的织构性质
Table 1 Textural property of samples
Sample BET surface
area A/ (m2·g−1)Pore volumea
v/ (cm3·g−1)Average pore
sizeb
d/nmg-C3N4 26.82 0.090 23.00 OH-g-C3N4 19.06 0.064 27.79 NH-g-C3N4 13.46 0.083 38.90 AM-g-C3N4 8.27 0.045 42.28 Fe/g-C3N4 46.49 0.233 49.08 Fe/OH-g-C3N4 49.92 0.258 35.51 Fe/NH-g-C3N4 16.88 0.088 35.21 Fe/AM-g-C3N4 23.88 0.133 34.44 a: BJH adsorption pore volume; b: BJH adsorption average pore size. -
[1] FUJIMORI S, INOUE S, Carbon monoxide in main-group chemistry[J]. J Am Chem Soc, 2022, 5(144): 2034–2050. [2] 刘赛赛, 姚金刚, 陈冠益, 等. 合成气一步法制备低碳烯烃和液体燃料催化剂研究进展[J]. 燃料化学学报 (中英文),2023,51(1):34−51.LIU Sai-sai, YAO Jin-gang, CHEN Guan-yi, et al. One-step catalyst for the preparation of light olefins and liquid fuels from syngas[J]. J Fuel Chem Technol,2023,51(1):34−51. [3] CHENG K, GU B, LIU X L, et al. Direct and highly selective conversion of synthesis gas into lower olefins: Design of a bifunctional catalyst combining methanol synthesis and carbon-carbon coupling[J]. Angew Chem Int Ed,2016,55(15):4725−4278. doi: 10.1002/anie.201601208 [4] JIAO F, LI J J, PAN X L, et al, Selective conversion of syngas to light olefins[J]. Science, 2016, 6277(351): 1065–1068. [5] JIAO F, PAN X L, GONG K, et al. Shape-selective zeolites promote ethylene formation from syngas via a ketene intermediate[J]. Angew Chem Int Ed,2018,57(17):4692−4696. doi: 10.1002/anie.201801397 [6] ZHANG P P, TAN L, YANG G H, et al. One-pass selective conversion of syngas to paraxylene[J]. Chem Sci,2017,8(12):7941−7946. doi: 10.1039/C7SC03427J [7] ZHAO B, ZHAI P, WANG P F, et al. Direct transformation of syngas to aromatics over Na-Zn-Fe5C2 and hierarchical HZSM-5 tandem catalysts[J]. Chem,2017,3(2):323−333. doi: 10.1016/j.chempr.2017.06.017 [8] YU X F, ZHANG J L, WANG X, et al. Fischer-Tropsch synthesis over methyl modified Fe2O3@SiO2 catalysts with low CO2 selectivity[J]. Appl Catal B: Environ,2018,232:420−428. doi: 10.1016/j.apcatb.2018.03.048 [9] XU Y F, LI X Y, GAO J H, et al. A hydrophobic FeMn@Si catalyst increases olefins from syngas by suppressing C1 by-products[J]. Science,2021,371(6529):610−613. doi: 10.1126/science.abb3649 [10] 马龙, 张玉玺, 高新华, 等. Fe3O4@PI催化剂的制备及其费托合成性能[J]. 燃料化学学报,2020,48(7):813−820. doi: 10.1016/S1872-5813(20)30057-8MA Long, ZHANG Yu-xi, GAO Xin-hua, et al. Preparation of Fe3O4@PI and its catalytic performance in Fischer-Tropsch synthesis[J]. J Fuel Chem Technol,2020,48(7):813−820. doi: 10.1016/S1872-5813(20)30057-8 [11] FANG W, WANG C T, LIU Z Q, et al. Physical mixing of a catalyst and a hydrophobic polymer promotes CO hydrogenation through dehydration[J]. Science,2022,377(6604):406−410. doi: 10.1126/science.abo0356 [12] OKOYE-CHINE C G, MOYO M, HILDEBRANDT D. The influence of hydrophobicity on Fischer-Tropsch synthesis catalysts[J]. Rev Chem Eng,2022,38(5):477−502. doi: 10.1515/revce-2020-0037 [13] CHEN Z, ZHANG J, ZHENG S K, et al. The texture evolution of g-C3N4 nanosheets supported Fe catalyst during Fischer-Tropsch synthesis[J]. Mol Catal,2018,444:90−99. doi: 10.1016/j.molcata.2016.12.011 [14] PARK H, YOUN D H, KIM J Y, et al. Selective formation of hägg iron carbide with g-C3N4 as a sacrificial support for highly active Fischer-Tropsch synthesis[J]. ChemCatChem,2015,7(21):3488−3494. doi: 10.1002/cctc.201500794 [15] KOO H M, WANG X, KIM A R, et al. Effects of self-reduction of Co nanoparticles on mesoporous graphitic carbon-nitride to CO hydrogenation activity to hydrocarbons[J]. Fuel,2021,287:119437. [16] ZHANG Y X, GUO X Y, LIU B, et al. Surface modification of g-C3N4-supported iron catalysts for CO hydrogenation: Strategy for product distribution[J]. Fuel,2021,305:121473. doi: 10.1016/j.fuel.2021.121473 [17] WANG Z Y, GUAN W, SUN Y J, et al. Water-assisted production of honeycomb-like g-C3N4 with ultralong carrier lifetime and outstanding photocatalytic activity[J]. Nanoscale,2015,7(6):2471−2479. doi: 10.1039/C4NR05732E [18] JÜRGENS B, IRRAN E, SENKER J, et al. Melem (2, 5, 8-Triamino-tri-striazine), an important intermediate during condensation of melamine rings to graphitic carbon nitride: Synthesis, structure determination by X-ray powder diffractometry, Solid-State NMR, and theoretical studies[J]. J Am Chem Soc,2003,125(34):10288−10300. doi: 10.1021/ja0357689 [19] GUO F, WANG L J, SUN H R, et al. A one-pot sealed ammonia self-etching strategy to synthesis of N-defective g-C3N4 for enhanced visible-light photocatalytic hydrogen[J]. Int J Hydrogen Energ,2020,45(55):30521−30532. doi: 10.1016/j.ijhydene.2020.08.080 [20] KANG S F, HE M F, CHEN M Y, et al. Surface amino group regulation and structural engineering of graphitic carbon nitride with enhanced photocatalytic activity by ultrafast ammonia plasma immersion modification[J]. ACS Appl Mater Interfaces,2019,11(16):14952−14959. doi: 10.1021/acsami.9b01068 [21] WANG X H, NAN Z D. Highly efficient Fenton-like catalyst Fe-g-C3N4 porous nanosheets formation and catalytic mechanism[J]. Sep Purif Technol, 2020, 233: 116023–116032 [22] AN S F, ZHANG G H, WANG T W, et al. High-density ultra-small clusters and single-atom Fe sites embedded in graphitic carbon nitride (g-C3N4) for highly efficient catalytic advanced oxidation processes[J]. ACS Nano,2018,12(9):9441−9450. doi: 10.1021/acsnano.8b04693 [23] DING Z X, CHEN X F, ANTONIETTI M, et al. Synthesis of transition metal-modified carbon nitride polymers for selective hydrocarbon oxidation[J]. ChemSusChem,2010,4(2):274−281. [24] ZHANG Y X, GUOX Y, LIU B, et al. Cellulose modified iron catalysts for enhanced light olefins and linear $ {\rm{C}}_5^+ $ α-olefins from CO hydrogenation[J]. Fuel,2021,294(9):120504.[25] MA J, SUN N, WANG C, et al. Facile synthesis of novel Fe3O4@SiO2@mSiO2@TiO2 core-shell microspheres with mesoporous structure and their photocatalytic performance[J]. J Alloy Compd,2018,743:456−463. doi: 10.1016/j.jallcom.2018.02.005 [26] TORSHIZI H O, POUR A N, MOHAMMADI A, et al. Fischer-Tropsch synthesis using a cobalt catalyst supported on graphitic carbon nitride[J]. New J Chem,2020,44(15):6053−6062. doi: 10.1039/D0NJ01041C [27] XU H, YAN J, XU Y G, et al. Novel visible-light-driven AgX/graphite-like g-C3N4 (X=Br, I) hybrid materials with synergistic photocatalytic activity[J]. Appl Catal B: Environ,2013,129:182−193. doi: 10.1016/j.apcatb.2012.08.015 [28] XIANG Q J, YU J G, JARONIEC M. Preparation and enhanced visible-light photocatalytic H2-production activity of graphene/C3N4 composites[J]. J Phys Chem C,2011,115(15):7355−7363. doi: 10.1021/jp200953k [29] LI Y P, ZHAN J, HUANG L Y, et al. Synthesis and photocatalytic activity of a bentonite/g-C3N4 composite[J]. RSC Adv,2014,4(23):11831−11839. doi: 10.1039/c3ra46818f [30] ZHANG S, GUO H Y, HOU W L, et al. Synthesis, structure and photocatalytic properties of benzo[ghi] perylenetriimide/graphitic carbon nitride composite[J]. Mater Lett,2018,221:38−41. doi: 10.1016/j.matlet.2018.03.045 [31] HUANG L Y, LI Y P, XU H, et al. Synthesis and characterization of CeO2/g-C3N4 composites with enhanced visible-light photocatatalytic activity[J]. RSC Adv,2013,3(44):22269−22279. doi: 10.1039/c3ra42712a [32] NIE C, ZHANG H T, MA H F, et al. Effects of Ce addition on Fe-Cu catalyst for Fischer-Tropsch synthesis[J]. Catal Lett,2019,149(5):1375−1382. doi: 10.1007/s10562-019-02700-2 [33] DING M Y, YANG Y, WU B S, et al. Study on reduction and carburization behaviors of iron phases for iron-based Fischer-Tropsch synthesis catalyst[J]. Appl Energy,2015,160:982−989. doi: 10.1016/j.apenergy.2014.12.042 [34] LU J Z, YANG L J, XU B L, et al. Promotion effects of nitrogen doping into carbon nanotubes on supported iron Fischer-Tropsch catalysts for lower olefins[J]. ACS Catal,2014,4(2):613−621. doi: 10.1021/cs400931z [35] YANG Q, WANG W Y, ZHAO Y X, et al. Metal-free mesoporous carbon nitride catalyze the Friedel-Crafts reaction by activation of benzene[J]. RSC Adv,2015,5(68):54978−54984. doi: 10.1039/C5RA08871B [36] CVIJOVIC L, KOTA K. Creating highly wettable paper towel-like aluminum surfaces through tuned bulk micro-manufacturing[J]. Int J Adv Manuf Technol,2018,98:2601−2609. doi: 10.1007/s00170-018-2290-5 [37] LI Z Z, MENG X C, ZHANG Z S. Fabrication of surface hydroxyl modified g-C3N4 with enhanced photocatalytic oxidation activity[J]. Catal Sci Technol,2019,9(15):3979−3993. doi: 10.1039/C9CY00550A [38] ORDOMSKY V V, LEGRAS B, CHENG K, et al. The role of carbon atoms of supported iron carbides in Fischer-Tropsch synthesis[J]. Catal Sci Technol,2015,5(3):1433−1437. doi: 10.1039/C4CY01631A [39] GUNASOORIYA G T K K, VAN BAVEL A P, KUIPERS H P C E, et al. Key role of surface hydroxyl groups in C-O activation during Fischer-Tropsch synthesis[J]. ACS Catal,2016,6(6):3660−3664. doi: 10.1021/acscatal.6b00634 [40] GU B, HE S, ZHOU W, et al. Polyaniline-supported iron catalyst for selective synthesis of lower olefins from syngas[J]. J Energy Chem,2017,26(4):608−615. doi: 10.1016/j.jechem.2017.04.009 -







 下载:
下载: